Articles
- Page Path
- HOME > Kosin Med J > Volume 37(1); 2022 > Article
-
Original article
The effects of ketamine on pain control in stage IV cancer patients receiving palliative care -
Seonghoon Kim1
 , Jihun Kang1,2
, Jihun Kang1,2 , Jongsoon Choi1
, Jongsoon Choi1 , Eunhee Kong1
, Eunhee Kong1
-
Kosin Medical Journal 2022;37(1):37-45.
DOI: https://doi.org/10.7180/kmj.21.003
Published online: March 14, 2022
1Department of Family Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
2Central Institute for Medical Research, Kosin University Gospel Hospital, Busan, Korea
- Corresponding author: Jihun Kang, MD, MS Department of Family Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, 262 Gamcheon-ro, Seo-gu, Busan 49267, Korea Tel: +82-51-990-6551 Fax: +82-51-990-3005 E-mail: josua85@naver.com
Copyright © 2022 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,390 Views
- 70 Download
- 2 Crossref
Abstract
-
Background
- We evaluated the effects of intravenous ketamine on cancer pain in stage IV cancer patients receiving palliative care.
-
Methods
- In total, 253 stage IV cancer patients with cancer pain hospitalized at a single tertiary hospital palliative care unit were included. The ketamine group contained 112 patients receiving ketamine, and the control group comprised 141 non-ketamine users. To evaluate the odds ratios (ORs) for favorable pain control, optimal pain control, and opioid-sparing effect among ketamine users, we used multivariable logistic regression adjusted for age and objective prognosis score. Differences in the visual analog scale (VAS) score, oral morphine equivalents, inter-dose frequency, and inter-dose amount were compared between both groups at the time of ketamine introduction (T0), after 24 hours (T1), and after 48 hours (T2) using repeated-measures analysis of covariance.
-
Results
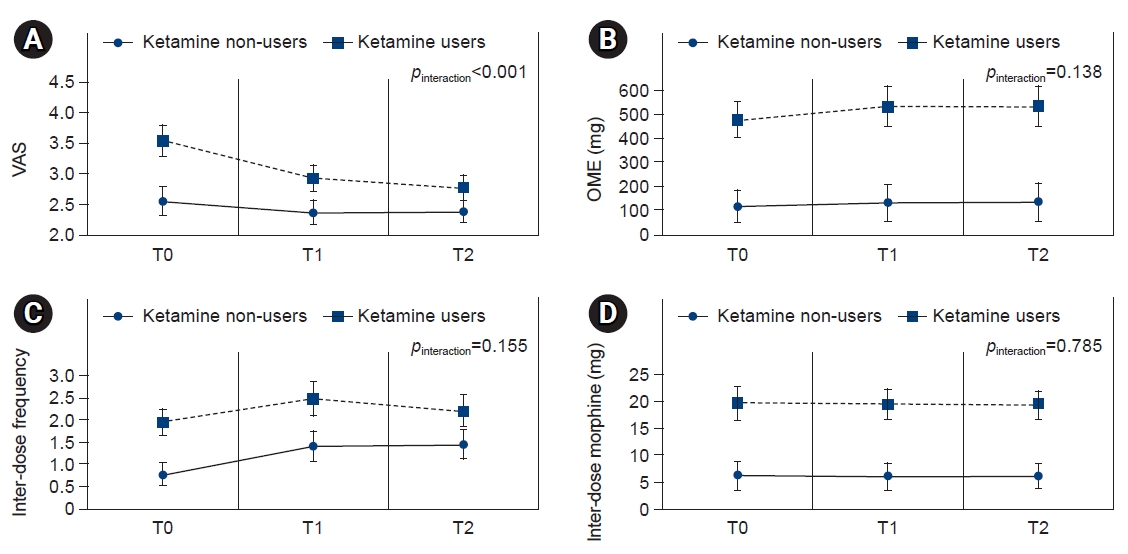
- The ketamine group was more likely to show favorable pain control (OR, 3.84; 95% confidence interval [CI], 1.76–8.37) and an optimal response (OR, 3.99; 95% CI, 1.73–9.22) than the control group. Compared to the control group, the ketamine group showed a higher VAS score at T0, but a more evident VAS score reduction at T1 and T2 (pinteraction<0.001). The ketamine group was less likely than the control group to experience depressive mood (OR, 0.31; 95% CI, 0.10–0.92), but had a higher risk of delirium (OR, 2.06; 95% CI, 1.12–3.81).
-
Conclusion
- Our findings suggest that ketamine can effectively reduce refractory cancer pain in stage IV cancer patients.
- The prevalence of cancer pain is estimated to be 50.7% in patients with cancer [1] and 70% in patients who have terminal cancer [2]. Refractory cancer pain refers to cancer-related pain that persists over time despite adequate administration of pain control medications, including morphine, and co-analgesics [3]. Conventional treatment cannot adequately relieve cancer pain in 20% to 40% of cases [4,5], and treatment of cancer pain can be challenging due to its complicated pathophysiology [6].
- Ketamine was mainly used as an anesthetic agent since the 1970s, but it began to gain interest as an effective analgesic when the N-methyl-D-aspartate (NMDA) receptor was discovered in the 1990s [6]. Ketamine blocks the NMDA receptor non-competitively, and analgesic effect is mediated by antagonism of the NMDA receptor [6,7]. Ketamine has gained interest in research fields because low dose ketamine may decrease the amount of opioid consumption and potentiate opioid activity [8,9]. Previous studies regarding the effects of ketamine on cancer pain showed conflicting results. A study from Hong Kong reported that ketamine use decreased the numerical rating scale score for pain from 7 to 4 [10]. Another study from China reported that the intensity of refractory cancer pain was decreased by half among 47% of patients with terminal cancer who used ketamine [10]. Furthermore, ketamine demonstrated superior pain relief effects in peripheral neuropathy compared to lidocaine or placebo [11]. Use of ketamine also showed beneficial effects on various types of pain including chronic and neuropathic pain [11,12]. However, a randomized controlled study conducted in Australia found that ketamine had no additional clinical effect on cancer pain [13].
- Although several studies on ketamine have been conducted, there is still a debate regarding the effectiveness of ketamine in the management of cancer pain. Although a randomized controlled study of Australia reported null effect of ketamine on controlling cancer pain, a majority of study participants were composed of lung and prostate cancer patients. Therefore, it is an area of uncertainty whether ketamine has a pain relief effect in terminal stomach and pancreatic cancer which are prevalent in Korea. In addition, despites a modest volume of evidence indicating ketamine might be effective in controlling terminal cancer pain in Asian population, few studies have estimated the pain relief effects of ketamine among patients with terminal cancer in Korea. Therefore, we aimed to evaluate the effect of intravenous ketamine on cancer pain and opioid-sparing among stage IV cancer patients at a palliative care unit.
Introduction
- All study protocols complied with the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of Kosin University Medical School (KUGH-2020-03-041), and the requirement of informed consent was waived.
- 1. Participants and study design
- We retrospectively assessed 343 stage IV cancer patients with cancer pain who had been hospitalized from January 2015 to February 2019 at a single tertiary hospital palliative care unit. Patients who died within 48 hours after using ketamine, or had missing data on ketamine use or cancer-related variables (n=90) were excluded. Thus, a total of 253 patients were included in the study analysis. Of the 253 patients, we categorized 112 patients receiving ketamine into a ketamine group and 141 non-ketamine users into a control group. We defined stage IV cancer patients as patients who had been diagnosed clinically or histopathology with cancer and showed evidence of distant metastasis.
- As the general pain control protocol for our palliative unit was administration of intravenous morphine, patients who were already taking oral opioids were administered intravenous morphine equivalent to one-third of the oral dose. For patients who started opioid therapy at the palliative care unit for the first time, the starting dose of intravenous morphine was 10 mg, and the daily dose was gradually increased until cancer pain was controlled with an inter-dose for ≤4 times a day. An inter-dose of intravenous morphine was one-fifth of the regular-opioid dose and was administered when cancer pain was not relieved with the regular opioid dose. The minimum interval between administrations was 2 hours, but there were no other limits.
- Patients with unrelieved cancer pain despite an administration of rapid-acting morphine (20% of regular regular-opioid dose) and dose escalation of regular opioid (50%–100% increment of previous regular opioid dose) were selected for the ketamine group, and 0.5–1 mg/kg/day of ketamine was given.
- 2. Data collection and measurements
- Data on the general characteristics and cancer-related variables such as the time since cancer diagnosis, cancer stage, and occurrence of metastasis were gathered from patients' electronic medical records by two trained medical data managers. A supervisor then reviewed and validated the data. Cancer stage was classified according to the American Joint Committee on Cancer staging system, 7th edition.
- Data were collected for 3 days from the first use of ketamine. Each case report contained information on general characteristics, such as anthropometric measurement, and information about the cancer type, disease duration, pain characteristics, and adverse effects profiles. Two independent reviewers verified the information against the medical records. Pain levels were routinely measured by trained medical personnel using the visual analog scale (VAS). The morphine doses used in the study are expressed as the oral morphine equivalent (OME). The conversion ratio of various morphine equivalents was as follows: oral morphine 100=intravenous morphine 33=transdermal fentanyl 1=intravenous fentanyl 1=oral methadone 20=intravenous methadone 16=oral oxycodone 70=transdermal buprenorphine 1.3 [14]. Data regarding ketamine, co-analgesics, and sedation drug prescriptions were gathered and dichotomously categorized into two groups (yes or no). Body mass index was calculated as weight in kilograms and height in meters (kg/m2) and divided into three groups according to criteria defined for East Asian populations (<23, 23–24.9, and ≥25 kg/m2) [15]. Smoking status was categorized into two groups (smokers or non-smokers), and alcohol drinking status was categorized into two groups (yes or no).
- The objective prognosis score (OPS) is a tool for predicting the survival of terminal cancer patients in palliative care units in Korea that was developed by Jho et al. [16], and it ranges from 0 to 8 points. In a study of terminal cancer patients admitted to palliative care units in Korea, Jho et al. [16] reported that the sensitivity, specificity, and overall accuracy of OPS with 3 as the cutoff to predict the 3-week survival rates were 74.7%, 76.5%, and 75.5%, respectively. The OPS was assessed by palliative care medical personnel on the first day of admission.
- 3. Study outcomes
- The change in pain intensity, OME, and inter-dose frequency at the time of ketamine introduction (T0), after 24 hours (T1), and after 48 hours (T2) were serially assessed. The primary study outcomes were the change in pain level between T0 and T2. A favorable response and optimal response to ketamine were considered when the pain intensity decreased by ≥2 points and ≥50%, respectively, at 48 hours after the introduction of ketamine [17].
- The secondary study outcomes were a reduction in OME, inter-dose frequency, and inter-dose amounts. Since there is no defined dose cutoff for opioid-sparing [18], we arbitrarily defined opioid-sparing effect as a reduction in opioid dose due to the use of ketamine. No opioid-sparing effect was considered when the dose was maintained.
- Headache, constipation, micturition associated difficulties, nausea, depressive mood, anxiety, delirium, and dyspnea were defined as ketamine-associated adverse events. After ketamine administration, physicians regularly visited the patients to assess the adverse events via face-to-face interviews for 48 hours. Once patients were compliant with depression, patient health questionnaire-9 (PHQ-9) was used to evaluate depressive mood, and patients ≥10 points in PHQ-9 were determined having depressive mood.
- 4. Statistical analysis
- The general characteristics and cancer-related variables of ketamine users and non-users were compared using a t-test for continuous variables and chi-square test for categorical variables. To evaluated the odds ratio (OR) for favorable pain control, optimal pain control, and opioid-sparing effect among ketamine users, we used a multivariable logistic regression analysis that was adjusted for age and OPS. Differences in VAS score, OME, inter-dose frequency, and inter-dose amount were compared between the ketamine group and control group at T0, T1, and T2 using repeated analysis of covariance.
- All tests were two-tailed, and a p-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA).
Methods
- 1. Clinical characteristics
- The general characteristics of the study participants are presented in Table 1. Two hundred and fifty-three patients (145 males and 108 females) were included. The mean age of the control group (67.4 years) was higher than that of the ketamine group (61.5 years). The most prevalent cancer type was lung cancer, followed by stomach and pancreatic cancer. The mean length of hospital stay of the ketamine group and control group was 23.2 days and 24 days, respectively. The ketamine group had a higher OPS than did the control group, and the OPS ≥2 was 62% in the ketamine group and 46.1% in the control group. The ketamine group was more likely to use co-analgesic (39.3%) and sedative drugs (70.5%) than was the control group (5% and 34.8%, respectively). Visceral pain was the most prevalent type of pain, followed by musculoskeletal and somatic pain. There were no significant differences in sex, body mass index, cancer diagnosis, time since cancer diagnosis, smoking status, alcohol drinking status, length of hospital stay, sleep time, or types of opioid between the two groups.
- 2. Efficacy: pain control
- The OR for favorable pain control, optimal pain control, and opioid-sparing effect are displayed in Table 2. While the ketamine group showed more favorable (OR, 3.84; 95% confidence interval [CI], 1.76–8.37) and optimal responses (OR, 3.99; 95% CI, 1.73–9.22) compared to the control group, there was no significant difference in opioid-sparing effect between the two groups.
- Changes in VAS score, OME, inter-dose frequency, and inter-dose amount are presented in Fig. 1.
- Although the VAS score of the ketamine group was higher than that of the control group at T0, the degree of VAS score reduction was more evident in the ketamine group than in the control group both at T1 and T2. However, OME, inter-dose frequency, and inter-dose amount was not significantly different between the ketamine group and the control group.
- 3. Tolerance: adverse effects
- Adverse effect profiles associated with ketamine use are presented in Table 3. The most common adverse event in the ketamine group was dyspnea (n=90), followed by nausea (n=68) and delirium (n=64). Participants in the ketamine group were less likely to complain of depressive mood (OR, 0.31; 95% CI, 0.10–0.92) compared to the control group, whereas, the ketamine group showed higher risk for delirium (OR, 2.06; 95% CI, 1.12–3.81). There were no statistically significant differences in other adverse events between the two groups.
Results
- This retrospective study included 253 patients with terminal cancer pain. Our results suggest that patients who received parenteral ketamine treatment at the palliative care unit showed a decrease in VAS score associated with ketamine use. In addition, the ketamine group had a higher likelihood for favorable and optimal response compared to the control group. Although the ketamine group was less likely to experience depressive mood, their risk for delirium was higher compared to that of the control group. There was no difference in other adverse events between the two groups.
- This study showed that ketamine was associated with decreased VAS score in end-stage cancer patients, and this result was consistent with those of previous studies. Cheung et al. [10] reported that 74.3% of patients (n=52) with refractory cancer pain successfully responded to ketamine. Another randomized, controlled, double-blind study reported that ketamine use reduced refractory cancer pain effectively in most patients [7]. Furthermore, Jackson et al. [2] showed in an open-label study that 67% of patients with refractory cancer pain from four palliative care units successfully responded to ketamine. The beneficial effects of ketamine on controlling cancer pain were also supported by a systemic review arguing that ketamine might be a viable treatment option for refractory cancer pain [19].
- However, the efficacy of ketamine in the management of cancer pain is still controversial. A blinded, randomized, controlled study of 185 adult cancer patients with cancer pain by Hardy et al. [13] reported that ketamine administration over 5 days showed no clinical advantage over placebo with increased adverse events. In addition, an earlier systemic review reported that there is limited evidence on ketamine being effective for controlling refractory cancer pain [20]. A recent systematic review also argued that there is insufficient evidence to conclude on the efficacy of ketamine for patients with refractory cancer pain [21]. The difference in pain control results among the previous studies might be due to different study populations, heterogeneous study settings, and inconsistent outcome definitions.
- We found that there was no difference in OME and inter-dose reduction between the two groups; therefore, no opioid-sparing effect was observed with ketamine use in our study. Reduced pain intensity after ketamine administration did not lead to a reduction in the administration of opioids because patients were reluctant to reduce their opioid dose as they were satisfied with their current pain medications. A small sample study reported that patients are more concerned about increasing pain that occurs when opioids are reduced than about the risk of overusing them [22]. Furthermore, based on the Centers for Disease Control and Prevention guidelines, physicians are recommended to reduce opioid dosage by 10% a month, so 48 hours may not have been enough time to observe significant opioid dose reduction [23].
- Mercadante et al. [24] reported that ketamine had no opioid-sparing effect, and this finding was in line with our findings. However, several previous studies observed opioid-sparing effects related to ketamine use. Fitzgibbon and Viola [25] found a 25% median opioid dose reduction, and Courade et al. [6] also reported opioid-sparing effects (10.5%). However, the number of participants in these studies and the number of patients showing opioid-sparing effects were small. Therefore, further studies are needed.
- We observed that the ketamine was less likely to experience depressive mood than was the control group. In line with our study finding, a recent narrative review reported that ketamine has antidepressant effect with a rapid onset time (within 24 hours) [26]. However, compared to that in the control group, the risk of delirium was elevated in participants who received ketamine. Although several studies have suggested that ketamine use might reduce postoperative delirium [27,28], a study reported that ketamine use might be a possible cause of delirium in cancer patients [29]. Considering the mechanism of action of ketamine, it is plausible to consider safety issues regarding psychological conditions with ketamine use. However, previous studies by Cheung et al. [10] and Mercadante et al. [7] showed tolerable safety profiles of ketamine. In addition, Sheehy et al. [30] investigated the frequency of adverse psychological events, such as hallucinations and changes in sleep patterns, with ketamine use and found no adverse events related to ketamine administration. However, it is still unclear whether the cause of delirium in our patients was the ketamine or cancer itself because delirium is prevalent in patients with terminal cancer.
- A few mechanisms can explain the pain control effects of ketamine. A previous study showed that ketamine acted as an antagonist at the NMDA receptor site, which is essential for central sensitization in pain [31]. By blocking this receptor, ketamine could exert its pain control effect [32]. Another possible mechanism is that the combined use of ketamine and morphine has a synergistic effect on pain control that was better than that of either drug alone. In an experimental study, Bossard et al. [9] reported that ketamine and morphine together significantly decreased the threshold of nociceptive flexion reflex compared to morphine or ketamine alone.
- This study had several limitations. First, since the study design was retrospective, and VAS scores were measured by medical personnel, VAS scores might be underestimated [33]. However, evaluation and documentation of pain are regularly performed using standardized assessment forms in our palliative care unit to ensure the quality of care. Second, because this study was conducted in stage IV cancer patients, the results of this study cannot be generalized to all cancer patients. Third, while there was consistency regarding the indication of ketamine use, the timing of the administration and ketamine dose could vary.
- In conclusion, this study suggests that ketamine can effectively decrease refractory cancer pain in patients with stage IV cancer. Further prospective studies with a larger sample size should be conducted in the future to confirm and expand on these findings.
Discussion
-
Conflicts of interest
Jihun Kang is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
-
Funding
None.
-
Author contributions
Conceptualization: SK, JK. Data curation: SK, JK. Formal analysis: SK, JK. Methodology: SK, JK. Visualization: SK, JK. Writing - original draft: SK, JK. Writing - review & editing: SK, JK, JC, EK. Approval of final manuscript: all authors.
Article information
| Characteristics | Total (n=253) | Ketamine group (n=112) | Control group (n=141) | p-value |
|---|---|---|---|---|
| Sex | 0.665 | |||
| Male | 145 (57.3) | 62 (55.4) | 83 (58.9) | |
| Female | 108 (42.7) | 50 (44.6) | 58 (41.1) | |
| Age (yr) | 64.8±11.6 | 61.5±11.3 | 67.4±11.2 | <0.001 |
| Body mass indexa) | 0.656 | |||
| <23 kg/m2 | 103 (40.7) | 48 (42.9) | 55 (39.0) | |
| 23–24.9 kg/m2 | 23 (9.1) | 15 (13.4) | 8 (5.7) | |
| ≥25 kg/m2 | 11 (4.3) | 5 (4.5) | 6 (4.3) | |
| Data not provided | 116 (45.8) | 44 (39.3) | 72 (51.1) | |
| Diagnosisb) | 0.886 | |||
| Lung cancer | 40 (15.8) | 11 (9.8) | 29 (20.6) | |
| Stomach cancer | 36 (14.2) | 15 (13.4) | 21 (14.9) | |
| Pancreatic cancer | 34 (13.4) | 21 (18.8) | 13 (9.2) | |
| Rectal cancer | 23 (9.1) | 12 (10.7) | 11 (7.8) | |
| Hepatocellular carcinoma | 20 (7.9) | 5 (4.5) | 15 (10.6) | |
| Others | 100 (39.5) | 48 (42.9) | 52 (36.9) | |
| Time since cancer diagnosis | 0.768 | |||
| <1 yr | 101 (39.9) | 44 (39.3) | 57 (40.4) | |
| 1–3 yr | 83 (32.8) | 37 (33.0) | 46 (32.6) | |
| ≥3 yr | 69 (27.3) | 31 (27.7) | 38 (27.0) | |
| Smoking status | 0.821 | |||
| Smoker | 47 (18.6) | 22 (19.6) | 25 (17.7) | |
| Non-smoker | 206 (81.4) | 90 (80.4) | 116 (82.3) | |
| Alcohol consumption | 0.848 | |||
| Yes | 45 (17.8) | 21 (18.8) | 24 (17.0) | |
| No | 208 (82.2) | 91 (81.2) | 117 (83.0) | |
| Length of hospital stay (day) | 23.7±43.8 | 23.2±38.7 | 24.0±47.7 | 0.537 |
| Objective prognosis scorec) | 0.009 | |||
| 0 | 20 (7.9) | 3 (2.7) | 17 (12.1) | |
| 1 | 99 (39.1) | 40 (35.7) | 59 (41.8) | |
| 2 | 101 (39.9) | 53 (47.3) | 48 (34.0) | |
| ≥3 | 33 (13.0) | 16 (14.3) | 17 (12.1) | |
| Co-analgesic (Keromin) use | <0.001 | |||
| Yes | 51 (20.2) | 44 (39.3) | 7 (5.0) | |
| No | 202 (79.8) | 68 (60.7) | 134 (95.0) | |
| Sedation drug use | <0.001 | |||
| Yes | 128 (50.6) | 79 (70.5) | 49 (34.8) | |
| No | 125 (49.4) | 33 (29.5) | 92 (65.2) | |
| Sleep time (hr) | 6.0±0.7 | 6.0±0.7 | 6.0±0.7 | 0.954 |
| Type of pain | 0.030 | |||
| Somatic | 1 (0.4) | 0 | 1 (0.8) | |
| Visceral | 245 (96.8) | 112 (100) | 133 (94.3) | |
| Musculoskeletal | 7 (2.8) | 0 | 7 (5.0) | |
| Type of opioid | 0.074 | |||
| Intravenous | 237 (93.7) | 103 (92.0) | 134 (95.0) | |
| Patch | 5 (2.0) | 0 | 5 (3.5) | |
| Intrathecal | 11 (4.3) | 9 (8.0) | 2 (1.4) |
Values are presented as number (%) or mean±standard deviation.
p-values were calculated using the t-test for continuous variables and the chi-square test for categorical variables.
a) Body mass index was categorized based on criteria tailored for East Asian populations.
b) The stage of cancer was IV and was categorized according to the American Joint Committee on Cancer staging system, seventh edition.
c) The objective prognosis score is a tool for predicting the survival of terminal cancer patients in palliative care units in Korea that was developed by Jho et al. [16], and it ranges from 0 to 8 points.
|
Control group (n=141) |
Ketamine group (n=112) |
p-value | |||
|---|---|---|---|---|---|
| No. (%) | OR | No. (%) | OR (95% CI) | ||
| Favorable responsea) | 11 (7.8) | Reference | 28 (25.0) | 3.84 (1.76–8.37) | 0.001 |
| Optimal responseb) | 9 (6.4) | Reference | 25 (22.3) | 3.99 (1.73–9.22) | 0.001 |
| Opioid-sparing effectc) | 76 (53.9) | Reference | 52 (46.4) | 0.64 (0.38–1.09) | 0.101 |
OR, odds ratio; CI, confidence interval.
p-values were calculated using a multivariable logistic regression after adjusting for age and the objective prognosis score.
a) A favorable response to ketamine was defined as a reduction in pain intensity by ≥2 points at 48 hours after the introduction of ketamine.
b) An optimal response was defined as a reduction in the pain intensity by ≥50% at 48 hours after the introduction of ketamine.
c) Since there is no dose cutoff for opioid-sparing, we arbitrarily defined an opioid-sparing effect as a reduction of the opioid dose due to ketamine.
Values are presented as number (%) unless otherwise indicated. Adverse effects were observed for 48 hours after ketamine administration.
OR, odds ratio; CI, confidence interval.
p-values were calculated using the chi-square test for categorical variables or multivariable logistic regression for ORs (age and objective prognosis score were adjusted in the multivariable logistic analysis).
- 1. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 2016;51:1070–90.ArticlePubMed
- 2. Jackson K, Ashby M, Martin P, Pisasale M, Brumley D, Hayes B. “Burst” ketamine for refractory cancer pain: an open-label audit of 39 patients. J Pain Symptom Manage 2001;22:834–42.ArticlePubMed
- 3. Currow DC, Spruyt O, Hardy J. Defining refractory pain in cancer for clinicians and researchers. J Palliat Med 2012;15:5–6.ArticlePubMed
- 4. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2–15.Article
- 5. Twycross R, Harcourt J, Bergl S. A survey of pain in patients with advanced cancer. J Pain Symptom Manage 1996;12:273–82.ArticlePubMed
- 6. Courade M, Bertrand A, Guerrini-Rousseau L, Pagnier A, Levy D, Lervat C, et al. Low-dose ketamine adjuvant treatment for refractory pain in children, adolescents and young adults with cancer: a pilot study. BMJ Support Palliat Care 2019 May 31 [Epub]. https://doi.org/10.1136/bmjspcare-2018-001739Article
- 7. Mercadante S, Arcuri E, Tirelli W, Casuccio A. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage 2000;20:246–52.ArticlePubMed
- 8. Kissin I, Bright CA, Bradley EL Jr. The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg 2000;91:1483–8.ArticlePubMed
- 9. Bossard AE, Guirimand F, Fletcher D, Gaude-Joindreau V, Chauvin M, Bouhassira D. Interaction of a combination of morphine and ketamine on the nociceptive flexion reflex in human volunteers. Pain 2002;98:47–57.ArticlePubMed
- 10. Cheung KWA, Chan PC, Lo SH. The use of ketamine in the management of refractory cancer pain in a palliative care unit. Ann Palliat Med 2020;9:4478–89.ArticlePubMed
- 11. Kvarnstrom A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand 2003;47:868–77.ArticlePubMed
- 12. Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage 2000;20:358–73.ArticlePubMed
- 13. Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol 2012;30:3611–7.ArticlePubMed
- 14. Mercadante S, Ferrera P, Villari P, Casuccio A, Intravaia G, Mangione S. Frequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unit. J Pain Symptom Manage 2009;37:632–41.ArticlePubMed
- 15. Anuurad E, Shiwaku K, Nogi A, Kitajima K, Enkhmaa B, Shimono K, et al. The new BMI criteria for Asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335–43.ArticlePubMed
- 16. Jho HJ, Suh SY, Yoon SJ, Lee SS, Ahn HY, Yamaguchi T, et al. Prospective validation of the objective prognostic score for advanced cancer patients in diverse palliative settings. J Pain Symptom Manage 2016;52:420–7.ArticlePubMed
- 17. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58.ArticlePubMed
- 18. Michelet D, Hilly J, Skhiri A, Abdat R, Diallo T, Brasher C, et al. Opioid-sparing effect of ketamine in children: a meta-analysis and trial sequential analysis of published studies. Paediatr Drugs 2016;18:421–33.ArticlePubMed
- 19. Bredlau AL, Thakur R, Korones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med 2013;14:1505–17.ArticlePubMed
- 20. van den Beuken-van Everdingen MH, de Graeff A, Jongen JL, Dijkstra D, Mostovaya I, Vissers KC, et al. Pharmacological treatment of pain in cancer patients: the role of adjuvant analgesics, a systematic review. Pain Pract 2017;17:409–19.ArticlePubMed
- 21. Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev 2017;6:CD003351.ArticlePubMed
- 22. Frank JW, Levy C, Matlock DD, Calcaterra SL, Mueller SR, Koester S, et al. Patients’ perspectives on tapering of chronic opioid therapy: a qualitative study. Pain Med 2016;17:1838–47.ArticlePubMedPMC
- 23. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. JAMA 2016;315:1624–45.ArticlePubMedPMC
- 24. Mercadante S, Caruselli A, Casuccio A. The use of ketamine in a palliative-supportive care unit: a retrospective analysis. Ann Palliat Med 2018;7:205–10.ArticlePubMed
- 25. Fitzgibbon EJ, Viola R. Parenteral ketamine as an analgesic adjuvant for severe pain: development and retrospective audit of a protocol for a palliative care unit. J Palliat Med 2005;8:49–57.ArticlePubMed
- 26. Corriger A, Pickering G. Ketamine and depression: a narrative review. Drug Des Devel Ther 2019;13:3051–67.ArticlePubMedPMC
- 27. Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:651–7.ArticlePubMed
- 28. Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267–75.ArticlePubMedPMC
- 29. Wein S, Spruyt O, Chapman R. Ketamine as a possible cause of delirium. J Pharm Pract Res 2002;32:212–4.Article
- 30. Sheehy KA, Lippold C, Rice AL, Nobrega R, Finkel JC, Quezado ZM. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res 2017;10:787–95.ArticlePubMedPMC
- 31. Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 1983;79:565–75.ArticlePubMedPMC
- 32. Soto E, Stewart DR, Mannes AJ, Ruppert SL, Baker K, Zlott D, et al. Oral ketamine in the palliative care setting: a review of the literature and case report of a patient with neurofibromatosis type 1 and glomus tumor-associated complex regional pain syndrome. Am J Hosp Palliat Care 2012;29:308–17.ArticlePubMed
- 33. Seers T, Derry S, Seers K, Moore RA. Professionals underestimate patients’ pain: a comprehensive review. Pain 2018;159:811–8.ArticlePubMed
References
Figure & Data
References
Citations

- Prevalence of Pain and Factors Affecting it in Patients with Lung Cancer in Ilam
Elham Bastani, Mahsa Rizehbandi, Fariba Shokri
International Journal of Cancer Management.2024;[Epub] CrossRef - Is ketamine useful for pain management in patients with stage IV cancer?
Sung Eun Kim
Kosin Medical Journal.2022; 37(1): 1. CrossRef

 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite