Articles
- Page Path
- HOME > Kosin Med J > Volume 36(2); 2021 > Article
-
Case Reports
Nilotinib-Induced Acute Interstitial Nephritis during the Treatment of Chronic Myeloid Leukemia - Min Jeong Kim
-
Kosin Medical Journal 2021;36(2):153-160.
DOI: https://doi.org/10.7180/kmj.2021.36.2.153
Published online: December 31, 2021
Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea
- Corresponding Author: Min Jeong Kim, Department of Internal Medicine, Pusan National University Yangsan Hospital, 20, Geumo-ro, Beomeo-ri, Mulgeum-eup, Yangsan, 50612 Republic of Korea, Tel: +82-55-360-2393, Fax: +82-55-360-2393, E-mail: dittomjk@gmail.com
• Received: August 13, 2021 • Revised: October 15, 2021 • Accepted: October 18, 2021
Copyright © 2021 by Korean Association of Medical Journal Editors
Articles published in Kosin Medical Journal are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,049 Views
- 11 Download
Abstract
- Tyrosine kinase inhibitors (TKIs) are targeted therapy drugs that selectively inhibit protein kinases. Nephrotoxicity associated with TKIs is uncommon. We report a case of a 39-year-old man with acute kidney injury that developed after nilotinib treatment for chronic myeloid leukemia (CML). The renal function of the patient decreased during treatment with nilotinib but improved when treatment was discontinued due to neutropenia. However, the renal function of the patient deteriorated again with the reintroduction of nilotinib for treatment. A renal biopsy revealed acute interstitial nephritis (AIN). The patient had no history of comorbidities and medication causing renal injury. Finally, we diagnosed the patient with nilotinib-induced AIN. After switching to imatinib mesylate, the renal function of the patient stabilized without further deterioration. Our case indicates that nilotinib can be a potential cause of renal dysfunction by inducing AIN when renal function deteriorates in patients treated with nilotinib.
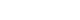
- A 39-year-old man with chronic-phase CML was referred to the nephrology clinic due to acute renal injury. The patient started treatment with nilotinib (800 mg/day) 4 months before the first visit to the nephrology clinic. The first line of defense for the patient in terms of medication was dasatinib. However, this was changed to nilotinib due to marrow suppression and poor overall response. The changes in serum creatinine levels after nilotinib treatment are shown in Figure 1. After initiating nilotinib treatment, the serum creatinine level was 1.06 mg/dL and glomerular filtration rate was 83.1 mL/min/1.73 m2. Proteinuria and hematuria were not observed. Four months after the start of treatment with nilotinib, the patient showed a good response with a bcr-abl1 major real-time quantitative polymerase chain reaction (IS-NCN) of 3.99%. Nine months after the start of treatment with nilotinib, the patient presented with neutropenia and acute kidney injury (serum creatinine 2.89 mg/dL). One month after discontinuation of nilotinib, renal function improved (serum creatinine 2.42 mg/dL), but it did not return to the baseline level of renal function before treatment for CML. The patient restarted nilotinib (400 mg/day) for treating CML. However, the patient presented with a rapid elevation of the serum creatinine level (to 4.80 mg/dL). Chemotherapy was terminated once again, and the patient was admitted to a nephrology clinic to identify the cause of the acute renal injury. The patient had no history of comorbidities and medication causing renal injury.
- On admission, his vital signs were as follows: blood pressure, 120/80 mmHg; pulse rate, 78/min; respiration rate, 20/min; and body temperature, 36.2 °C. Chest auscultation revealed no abnormalities. No significant findings were observed during abdominal examination, and no edema was found in the lower limbs. Blood test results were as follows: white blood cell count, 4100/mm3; hemoglobin, 9.7 g/dL; platelet count, 272 × 103/μL; sodium, 142 mEq/L; potassium, 4.3 mEq/L; chlorine, 112 mEq/L; blood urea nitrogen, 33.7 mg/dL; creatinine, 4.80 mg/dL; total protein, 7.4 g/dL; albumin, 4.3 g/dL; uric acid, 8.7 mg/dL; lactate dehydrogenase, 363 U/L; aspartate transaminase/alanine transaminase ratio, 10/8 IU/L; and C-reactive protein, 0.04 mg/dL. Additionally, IgA level was 146 mg/dL, IgG 1651 mg/dL, IgM 78 mg/dL, ASO (−), C3 119 mg/dL, C4 42 mg/dL, Fluorescent antinuclear antibody (−), and anti-dsDNA (−). Monoclonal gammopathy was not observed on serum or urine electrophoresis. The results of the urine test were as follows: specific gravity, 1.0111; pH, 5.5; urine protein (trace); spot urine protein/creatinine ratio, 250.1 mg/g; urine glucose (−); albuminuria (−); urine occult blood 3+; urine white blood cell count, 0–2/high power field; and urine red blood cell count, 21–29/ high-power field. Chest radiography revealed no significant findings. Abdominal ultrasound revealed normal-sized kidneys on both sides, with no significant findings (Fig. 2).
- A renal biopsy showed infiltration of lymphocytes and eosinophils at the renal interstitium and peritubular area along with tubular necrosis (Fig. 3). No infiltration of immune complex deposits was observed by immunofluorescence microscopy. Electron microscopy revealed foot process effacement, but abnormalities in the glomerular baseline membrane and subendothelial deposits were not observed.
- With the diagnosis of nilotinib-induced AIN, nilotinib was discontinued and intravenous fluid hydration was continued. Subsequently, the patient showed improved renal function after 3 months (serum creatinine level 2.05 mg/dL). Imatinib mesylate 100 mg/day was initiated for CML treatment. The dose of imatinib was gradually increased to 300 mg/day, and the renal function of the patient remained stable without further deterioration.
CASE
- TKIs have shown remarkable survival benefits in patients with CML, with fewer adverse effects. Four TKIs, namely imatinib, nilotinib, dasatinib, and bosutinib, have been approved for the treatment of CML by the US Federal Drug Administration (FDA) and the European Medicine Agency (EMA) as first-line choices.1 Nephrotoxicity is a rare side effect of TKI treatments, particularly nilotinib.3 A few studies have shown that imatinib has a higher prevalence of renal injury than nilotinib or dasatinib. Yilmaz et al. investigated the estimated glomerular filtration rate change in 466 newly diagnosed chronic phase CML patients treated with imatinib, dasatinib, or nilotinib for 12 months. They reported that there was a decline in the mean glomerular filtration rate only among patients treated with imatinib and dasatinib and not in those treated with nilotinib. 4 Ren et al. analyzed the incidence of chronic renal injury in CML patients treated with imatinib, nilotinib, or dasatinib. The authors found a higher incidence of chronic renal injury in patients treated with imatinib than in those treated with nilotinib or dasatinib.5 Marcolino et al. demonstrated that imatinib treatment duration is related to a decreased estimated glomerular filtration rate in patients with CML.6
- The mechanism of TKI-related renal injury is unknown. Imatinib can induce renal injury, which has been suggested to be associated with tumor lysis syndrome and tubular toxicity.3,4 Imatinib-induced tumor lysis syndrome causes acute renal injury that results from the precipitation of intracellular contents, such as uric acid and phosphate, released by rapid destruction of cells during cancer treatment of renal tubules.6 Tubular toxicity induced by imatinib was related to the PDGF-mediated pathway. Imatinib inhibits the PDGF-mediated pathway, which is critical for tubulogenesis after tubular injury.3
- Dasatinib is known to be related to proteinuria, rather than decreased renal function. A phase I dose-escalation and a pharmacokinetic study reported that 18% of the patients treated with dasatinib showed proteinuria.7 The mechanism of proteinuria related to dasatinib has been associated with the disruption of vascular endothelial growth factor (VEGF) signaling, which involves podocytes and endothelial cells in humans.8
- Nilotinib is metabolized mainly by cytochrome P450 3A4, and metabolites are excreted via the bile into feces and not into the urine. No dosage adjustment is needed in patients with renal impairment. 1,4 Renal dysfunction is a rare adverse reaction that develops during nilotinib treatment. None of the patients who participated in phase I and II trials of nilotinib for CML developed renal failure.7 To date, there have been only three case reports of nilotinib-induced renal injury, which were associated with tumor lysis syndrome in chronic phase CML patients.9 However, AIN from nilotinib treatment has not been described in the literature yet.
- AIN is a cause of acute renal injury. Drug-mediated interstitial nephritis accounts for 70%–90% of all interstitial nephritis cases. It has histological characteristics including inflammation and edema of the renal interstitium.10 Concerning TKI-induced interstitial nephritis, VEGF-targeting drugs such as sunitinib and sorafenib have been described in some case reports. Sunitinib- and sorafenib-induced interstitial nephritis is suggested to be linked to blocking the VEGF pathways that mediate a variety of signaling pathways.11
- In the present report, it is presumed that nilotinib might induce interstitial nephritis by blocking the BCR ABL. However, we believe that further research is needed to clarify the mechanism of nilotinib-induced interstitial nephritis.
- Recently, immune-related adverse effects have been reported in patients with immune checkpoint inhibitors (ICPIs) as a new type of chemotherapy line. ICPIs are monoclonal antibodies used for cancer therapy by blocking inhibitory receptors expressed on T cells. Various antibodies against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed death 1 protein (PD-1), and programmed death-ligand 1 (PD-L1) currently have been developed. Anti- CTLA-4 receptor antibodies promote the activity and proliferation of quiescent T-cells while anti-PD-1 receptor and anti-PD-L1 antibodies inhibit suppression of activated T-cells. The vigorous activated immune system is related to several immune- related adverse effects including nephrotoxicity. ICPI-associated acute kidney injury (AKI) has similar clinical and histological characteristics to other drug-induced acute interstitial nephritis, but with a long latency period. CPI-associated AKI might be related to a distinct mechanism of reprogramming of the immune system, through loss of tolerance with reactivation of drug-specific T cells induced by the immune checkpoint inhibitors.11 In case series of CPI-induced AKI, CPI discontinuation with corticosteroids initiation led to complete or partial improvement of the kidney.12 PD-1 receptor antibody-associated AKI can be induced by down-regulation of tolerance to the drug known to cause acute interstitial nephritis.13 CPI-associated AKI occurs relatively later than other drug-induced AKI. In one retrospective single-center study, the majority pathological finding of patients with CPI-associated AKI was acute interstitial nephritis. However it can frequently cooccur with other glomerular pathologies.14 A case series by Izzedine et al also reported that acute tubular injury, acute interstitial nephritis, and minimal change disease are the most common histological findings in renal injury associated with pembrolizumab, PD-1 receptor antibody.15 Because CPI-associated AKI lacks ideal biomarkers to distinguish it from other drug-induced AKI, renal biopsy is needed to identify the correct diagnosis.14
- In this case study, we report a case of biopsyproven interstitial nephritis in a 39-year-old patient with CML who was treated with a second-generation TKI (nilotinib). Our patient had microscopic hematuria without any manifestations of hypersensitivity, such as skin rash, eosinophilia, and fever. The renal function of the patient decreased during treatment with nilotinib but improved when nilotinib was discontinued due to neutropenic fever. However, the renal function of the patient deteriorated again with the reintroduction of nilotinib for CML treatment. Through kidney biopsy, we found that the cause of the decreased renal function was AIN. The patient had not taken any offending drugs that commonly result in drug-induced interstitial nephritis, except for nilotinib. Finally, we diagnosed the patient with nilotinib-induced AIN. After switching to imatinib mesylate, the patient’s renal function stabilized without further deterioration.
- Kidney injury associated with nilotinib can be underestimated because it is relatively uncommon. However, our case indicates that nilotinib can be a potential cause of renal dysfunction by inducing interstitial nephritis when renal function deteriorates following treatment with nilotinib. In patients diagnosed with nilotinib-induced interstitial nephritis, replacement with other TKIs could be considered to prevent further renal dysfunction, as in our case.
DISCUSSION
Fig. 1Changes in the level of serum creatinine after treatment course. The serum creatinine levels increased during the treatment with nilotinib and decreased after the discontinuation of nilotinib.

Fig. 2Ultrasonographic images of the kidney. A renal ultrasound revealed normal-sized kidneys on both sides with no significant findings.
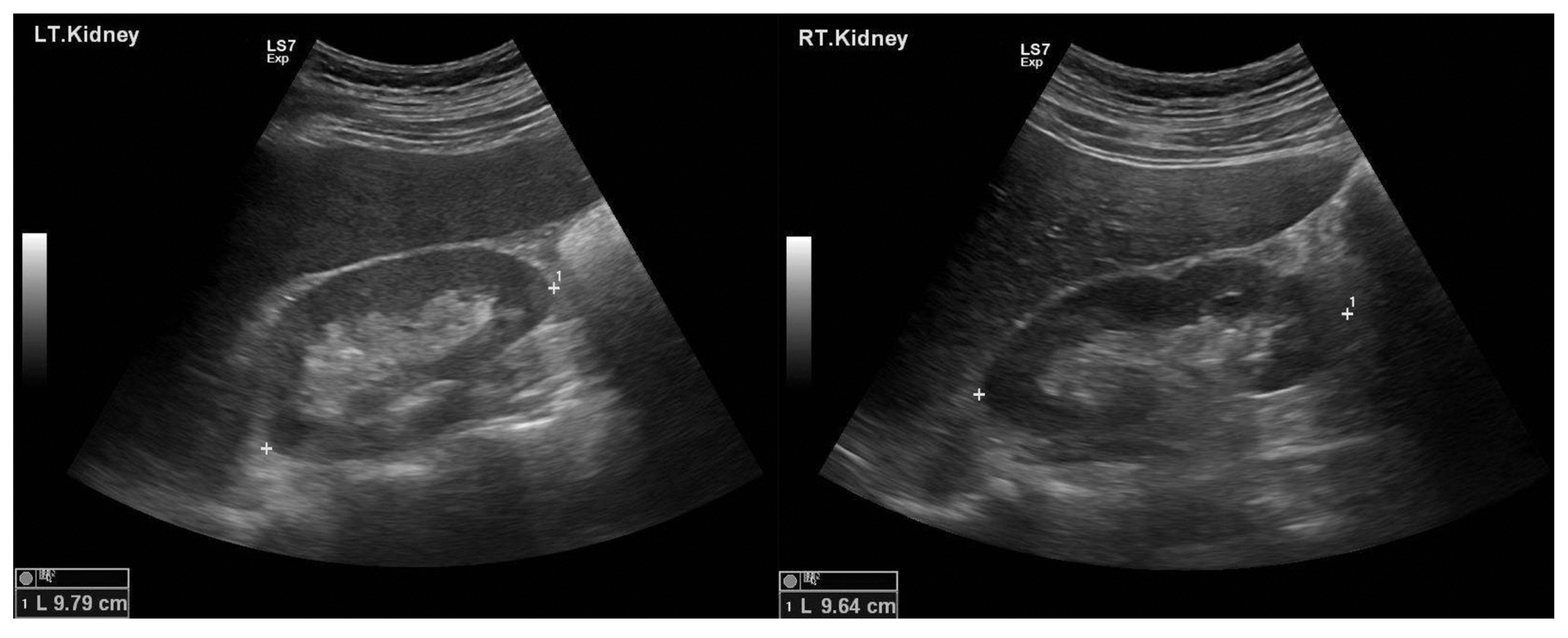

Fig. 3Histologic findings of the kidney. Infiltration of lymphocytes is present at the renal interstitium along with tubular necrosis (hematoxylin and eosin stain, ×100) (A). Eosinophils (black arrow) are seen at the renal interstitium (hematoxylin and eosin stain, ×400) (B).

- 1. Jabbour E, Kantarjian H, Cortes J. Use of Second- and Third-Generation Tyrosine Kinase Inhibitors in the Treatment of Chronic Myeloid Leukemia: An Evolving Treatment Paradigm. Clinical Lymphoma Myeloma and Leukemia 2015;15:323–4.Article
- 2. Blay JY, von Mehren M. Nilotinib: a novel, selective tyrosine kinase inhibitor. Semin Oncol 2011;38:Suppl 1. S3–9.ArticlePMC
- 3. Jhaveri KD, Wanchoo R, Sakhiya V, Ross DW, Fishbane S. Adverse Renal Effects of Novel Molecular Oncologic Targeted Therapies: A Narrative Review. Kidney Int Rep 2016;2:108–23.ArticlePubMedPMC
- 4. Yilmaz M, Lahoti A, O’Brien S, Nogueras-Gonzáez GM, Burger J, Ferrajoli A, et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 2015;121:3894–904.ArticlePubMed
- 5. Ren X, Qin Y, Huang X, Zuo L, Jiang Q. Assessment of chronic renal injury in patients with chronic myeloid leukemia in the chronic phase receiving tyrosine kinase inhibitors. Ann Hematol 2019;98:1627–40.ArticlePubMed
- 6. Marcolino MS, Boersma E, Clementino NCD, Macedo AV, Marx-Neto AD, Silva M, et al. Imatinib treatment duration is related to decreased estimated glomerular filtration rate in chronic myeloid leukemia patients. Ann Oncol 2011;22:2073–9.ArticlePubMed
- 7. Jabbour E, Lipton JH. A critical review of trials of first-line BCR-ABL inhibitor treatment in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Clin Lymphoma Myeloma Leuk 2013;13:646–56.ArticlePubMedPMC
- 8. Ozkurt S, Temiz G, Acikalin MF, Soydan M. Acute renal failure under dasatinib therapy. Ren Fail 2010;32:147–9.ArticlePubMed
- 9. Hua J, Iwaki Y, Inoue M, Hagihara M. Tumor lysis syndrome soon after treatment with hydroxyurea followed by nilotinib in two patients with chronic-phase chronic myelogenous leukemia. Int J Hematol 2013;98:243–6.ArticlePubMed
- 10. Praga M, Sevillano A, Auńón P, Gonzáez E. Changes in the aetiology, clinical presentation and management of AIN, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant 2015;30:1472–9.ArticlePubMed
- 11. Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med 2009;122:322–8.ArticlePubMed
- 12. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47.ArticlePubMedPMC
- 13. Shirali AC, Perazella MA, Gettinger S. Association of AIN with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016;68:287–91.PubMed
- 14. Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond interstitial nephritis: singlecenter experience. J Immunother Cancer 2019;7:2–14.PubMedPMC
- 15. Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, et al. Renal toxicities associated with pembrolizumab. Clin Kidney J 2018;12:81–8.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite