Articles
- Page Path
- HOME > Kosin Med J > Volume 38(4); 2023 > Article
-
Original article
Clinical outcomes of prostate artery embolization for management of benign prostate hyperplasia (prostate larger than 100 mL) with or without hematuria -
Soodong Kim

-
Kosin Medical Journal 2023;38(4):259-266.
DOI: https://doi.org/10.7180/kmj.23.122
Published online: November 7, 2023
Department of Urology, Dong-A University Hospital, Busan, Korea
- Correspondence Author: Soodong Kim, MD, PhD Department of Urology, Dong-A University Hospital, 26 Daesingongwon-ro, Seo-gu, Busan 49201, Korea Tel: +82-51-240-2673 Fax: +82-51-253-0591E-mail: urotan@dau.ac.kr
• Received: April 25, 2023 • Revised: May 30, 2023 • Accepted: June 5, 2023
Copyright © 2023 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 480 Views
- 10 Download
Abstract
-
Background
- In this study, we report 1-year follow-up clinical results of prostate artery embolization (PAE) in patients with glandular hematuria or acute urinary retention caused by a large prostate (over 100 mL).
-
Methods
- Twenty-one consecutive patients undergoing PAE from March 2018 to July 2020 were included in this retrospective study. Clinical follow-up was conducted for all patients 1, 3, 6, and 12 months after the procedure. The outcome measures included the International Prostate Symptom Score (IPSS), quality of life (QoL), peak urinary flow rate (Qmax), post-void residual (PVR), prostate volume, prostate-specific antigen, and complications. A p-value <0.05 was considered statistically significant.
-
Results
- Twenty-one patients with severe benign prostatic hyperplasia (BPH) with acute urinary retention or prostatic hematuria were enrolled in this study. Technical success rate was 90.5% (19/21), and unilateral PAE was done in 2/21 (9.5%) patients by pelvic vascular obliteration. In all patients, the mean IPSS, QoL score, Qmax, and PVR were significantly improved at 12 months post-PAE. The mean IPSS decreased from 26.1 to 12.1 points (p<0.05), mean QoL score decreased from 4.6 to 2.9 points (p<0.05), mean Qmax increased from 2.1 to 9.4 mL/s (p<0.05), and mean PVR decreased from 300.0 to 70.7 mL (p<0.05). The catheter was successfully removed from 19/21 patients and clinical success rate was 90.5%.
-
Conclusions
- PAE was an effective and safe treatment option for patients with BPH and very large prostates (>100 mL) and urinary retention or gross hematuria associated with BPH in men unfit for surgery.
- Benign prostatic hyperplasia (BPH) is a benign enlargement of prostate gland and is a major cause of lower urinary tract symptoms (LUTS) in men. Usually, BPH is not life-threatening condition, but it adversely affects quality of life (QoL). Among patients with BPH, LUTS were induced in approximately one in three men, and the clinical progression in 10% despite medication.
- Histologically at autopsy, the prevalence of BPH increased to 50%–60% in men in their 60s and gradually increased with age. As the aging society gradually enters, the prevalence of BPH is increasing [1]. So, the better treatment is necessary for growing number of elderly men [2]. Basically, BPH is treated with medical treatment (α-adrenoreceptor antagonists or 5α-reductase inhibitors). If medical treatment is ineffective, surgical treatment could be considered [3]. Although transurethral resection of the prostate (TURP) is the standard treatment for patient who do not respond to medical treatment, but some patients cannot tolerate TURP for medical (e.g., comorbidity) or technical (e.g., large prostate) reasons [4]. In response to this, interest in minimal invasive surgery with less morbidity is increasing [5-7].
- As the number of elderly patients increases and the number of comorbidities they have, the number of patients taking anticoagulants is increasing. Along with this phenomenon, the proportion of patients with enlarged prostate and hematuria is increasing. Prostatic origin gross hematuria usually resolved conservative measures but, in refractory hematuria cases especially in large prostate hyperplasia, prostate artery embolization (PAE) could be a good option [8].
- As a form of minimal invasive treatment, PAE treatment was introduced in 2010 [9]. PAE is an interventional radiological technique that directly occludes the prostate artery and causes prostate infarction. The effectiveness and safety of this technique have already been demonstrated following its initial clinical implementation. However, there are not much data on the clinical effect of PAE in cases of very large prostate (>100 mL). In this study, we report 1-year follow-up clinical results of PAE in patients with glandular hematuria or acute urinary retention (AUR) by large prostate (over 100 mL).
Introduction
- Ethical statements: This study was approved by the Institutional Review Board of Dong-A University Hospital (IRB No. DAUHIRB-19-026) and was conducted in accordance with the recent Declaration of Helsinki. Informed consent was waived.
- From January 2018 to December 2020, at a single-center, 21 consecutive patients who received PAE were retrospectively reviewed under an institutional review board approved protocol and ethical issues were considered [10].
- Included patients were who had gross hematuria or AUR due to larger than 100 mL prostate. All patients underwent evaluations of medical and surgical history, International Prostate Symptom Score (IPSS), QoL index questionnaire and Charlson Comorbidity Index. Peak urinary flow rate (Qmax) and post-voiding residual urine volume were recorded. Also, pre-procedure prostate-specific antigen (PSA) and prostate volume (PV; transrectal or transabdominal ultrasound) were obtained. The prostate biopsy was performed for distinguishing prostate cancer when PSA level was above 4.0 ng/dL. In patients with gross hematuria, urine cytology, ultrasound of the kidneys, ureters & Bladder, and cystoscopy were performed to discriminate urinary tract malignancy, also. Exclusion criteria for PAE included biopsy proven prostatic cancer, active prostatitis or urinary tract infection, previous surgical procedure or other invasive treatment for benign prostate hyperplasia, large bladder diverticula or bladder stones, and chronic renal failure.
- 1. Follow-up and outcome evaluation
- Clinical follow-up was done in all patients 1, 3, 6, and 12 months after the procedure. Outcome measures included IPSS, QoL, Qmax, post-void residual urine volume (PVR), PV, PSA, and complications. A p-value <0.05 was considered statistically significant. The technical success was defined as bilateral successful PAE. Clinical success was defined as improvement of LUTS. The LUTS was assessed by using IPSS and QoL questionnaires or self-voiding was possible in men with urinary retention prior to PAE. Clinical failure was considered a case where self-voiding was impossible after indwelling catheter removal.
- 2. Procedure
- Angiography and PAE were performed by one interventional radiologist on an inpatient basis at the interventional radiology suite (Allula Clarity FD 20; Philips Healthcare) equipped with the cone-beam computed tomograhpy (CBCT) option (XperCT; Philips Healthcare) after patients have signed informed consent. The procedure was performed via the right femoral arterial access under local anesthesia. Initial pelvic angiography was performed using a power injector to evaluate iliac vessels and the prostate arteries during arterial and late phases. Then, selective bilateral internal iliac arteriograms were obtained using a 5-French Robert’s uterine artery catheter (RUC catheter; Cook) in the anterior-posterior view. CBCT was performed with the 5-French RUC catheter located in the main trunk of internal iliac artery in the same tip of the catheter as was used with digital subtraction angiography to evaluate the origin of the prostate arteries. Prostate arteries for each side were identified by using vessel-tracking software (EmboGuide; Philips Healthcare) applied to the CBCT datasets. The software allowed the automated extraction of candidate vessels between a user-selected starting point (the catheter tip) and a segmented target (the prostate). Super-selection of each prostate artery was performed by using microcatheter (Veloute 1.7; Asahi) and microguidewire (Meister 0.016; Asahi) in the best aspect (ipsilateral oblique perspective, 25°–55°) to identify the prostate artery under the roadmap technique. Then, prostatic arterial digital subtraction angiography was performed by manual injection in the anterior-posterior and same ipsilateral oblique view. Embolization was performed using 250 to 355 non-spherical polyvinyl alcohol particles (Contour; Boston Scientific) in all patients. Contour was diluted in 20 mL of normal saline and 30 mL of contrast medium in a 2:3 solution. The mixture was slowly injected through 1-mL syringe under fluoroscopic guidance until we reached an end point of near stasis of contrast agent without reflux of embolic agent.
- 3. Statistical analysis
- The categorical variables were presented as numbers and frequencies and continuous variables were presented as mean±standard deviation or median and interquartile range. For trend analysis, repeated measure analysis of variance was used for IPSS, QoL, Qmax, PVR, PSA, and PV. All tests were two-sided, and p-values <0.05 were considered statistically significant. Analyses were performed using SPSS statistics v 18.0 for Window (IBM Corp.).
Methods
- Twenty-one patients with severe BPH with AUR or prostatic hematuria were enrolled in this study. Patients’ basic demographic data are presented in Table 1. Bilateral PAE was technically successful in 19 out of 21 patients (90.5%), and unilateral PAE was done in two out of 21 patients (9.5%) by pelvic vascular obliteration.
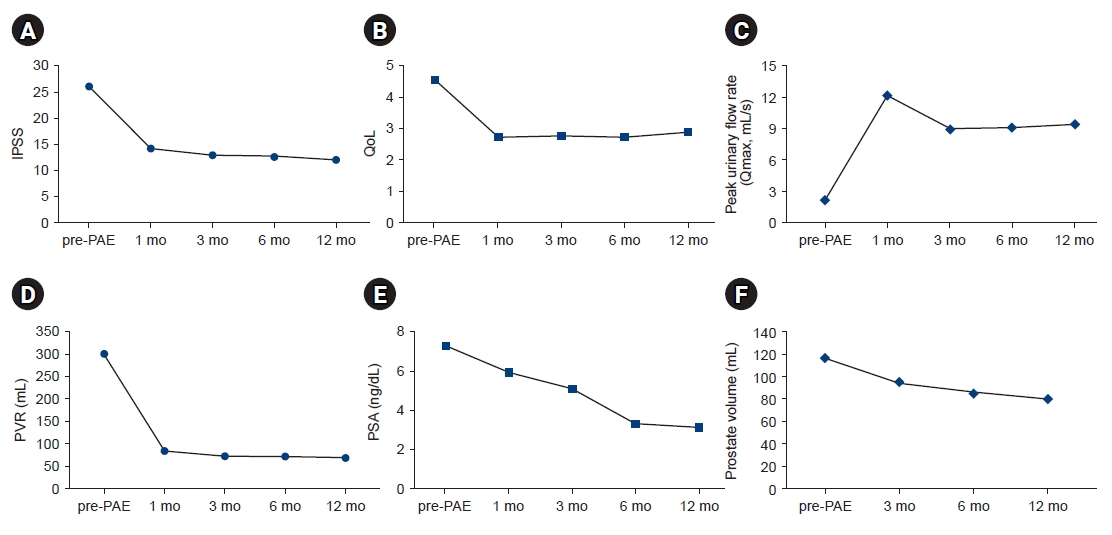
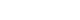
- Patients were followed for at least 12 months. Patient data before and after PAE are presented in Table 2. In all patients, the mean IPSS, QoL score, Qmax, and PVR were significantly improved at 12 months postoperatively (Table 2). The mean IPSS decreased from 26.1 to 12.1 points (p<0.05), mean QoL score decreased from 4.6 to 2.9 points (p<0.05), mean Qmax increased from 2.1 to 9.4 mL/s (p<0.05), and mean PVR decreased from 300.0 to 70.7 mL (p<0.05). Furthermore, the mean prostatic volume and mean PSA level decreased from 118.1 to 79.5 mL (mean reduction of 32.7%, p<0.05) and 7.4 ng/dL to 3.3 ng/dL (p<0.05), significantly (Fig. 1).
- The catheter was successfully removed from 19 of 21 patients and clinical success rate was 90.5%. Seventeen patients were able to remove the catheter after 1 week of the procedure. However, two patients needed additional 2 weeks of catheter placement. Median hospital stay was 2 days. Two patients could not void after PAE, they diagnosed as a detrusor underactivity by urodynamic study. Eight patients with hematuria had controlled of bleeding following the embolization in all patients.
- Adverse events were summarized in Table 3. There were no major complications (>3 Clavian complications) and nontarget embolization. Nine patients had minor complications. Four patients (19%) had a urinary tract infection, and they were controlled by 2 weeks of antibiotics. Two patients (9.5%) felt a burning sense of the perineum and they were controlled by nonsteroidal anti-inflammatory drug (NSAID) only. Three patients developed urge incontinence after removed catheter. They needed 2 to 3 diapers per day immediately after removed catheter for managing urge incontinence, but this symptom was resolved by conservative care with anticholinergics.
Results
- The patients with a prostate larger than 100 mL had limited treatment options. Traditionally, surgery (simple prostatectomy) has been considered as treatment of choice. But these have a relatively high risk of adverse events like that bleeding and postoperative incontinence [1,2]. In addition, endoscopic approach using Holium or Thulium laser (Holium laser enucleation of prostate and Thulium laser enucleation of prostate) has been widely used as the development of minimal invasive treatment. However, endoscopic laser enucleation also has many difficulties that necessity of anesthesia, bleeding, incontinence, urethral stricture, or retrograde ejaculation etc. in applying to all large BPH patients, and steep learning curve [11-14]. Nowadays PAE has been considered as another minimal invasive treatment of option for who could not be suited to surgery.
- Patients included in this study had difficulties in surgery or anesthesia due to comorbidity, 13 out of 21 (61.9%) patients taking anticoagulant due to previous coronal heart disease or cerebrovascular accident. And seven patients (33%) had gross hematuria, also.
- Recent papers have shown good results for the treatment of large prostates with PAEs, with prostate size reductions of 32% to 45% over 12 months, and 57% to 68% of the IPSS and QoL improved [15-19]. Feng et al. [20] performed a meta-analysis that reported the efficiency and safety of PAE and indicated that the IPSS and QoL scores showed great improvement after PAE (p<0.05). In present study, the PV reduction rate was 32.7% (from 118.1 to 79.5 mL) and IPSS decreased from 26 points to 12 points (–53.6%) after 12 months of observation. Besides these, other monitored functional results (QoL, PSA, post-voiding residual urine volume, and urine flow rate) were significantly improved at 12 months post-PAE, like the results reported by Gao et al. [21]. Furthermore, Lebdai et al. [22] reported mean IPSS reduction was 11.9 points at post-PAE 1 month, which was maintained post-PAE 6 months.
- In this study, IPSS and QoL at 1 month were improved and which was persisted for 12 months, also. PV showed a significant reduction at 3 months post-PAE, and it was maintained continuously for 12 months (Fig. 1). Pisco et al. [23] reported that the post-PAE PV reduction was related to clinical efficiency. These clinical results are presumed to be related to the histopathologic degeneration of the prostate by occluding the prostate vasculature [24].
- Additionally, a study investigating PAE specifically in catheter-dependent patients with large BPH showed 86% success in catheter removal in mean of 18.2 days after procedure [11]. In this study, mean catheterization date was 7.7 days and 90.4% (19/21) catheter free rate, also. Catheter were removed after 1 week of PAE procedure in outpatient clinic. After removal of the catheter, four patients could not void and maintained an additional catheterization for 2 weeks. After 2 weeks of additional catheterization, two patients were able to void, but two patients were still unable to void, and they needed intermittent catheterization. These patients were checked urodynamic study and diagnosed as detrusor underactivity.
- In our study, PAE was performed by a single radiologist who had enough experience, and 21 cases were performed successfully. Most studies used computed tomograhpy angiography or magnetic resonance angiography prior to the intervention to identify the prostatic artery [23,25-31], and CBCT was usually performed only to rule out nontarget embolization. We checked CBCT before embolization in all patients [31,32]. For achieving optimal outcome bilateral PAE is important [33]. CBCT can help reduce the risk of embolization by visualizing the prostate arteries to help identify collateral supply vessels. We defined technical success as accomplished bilateral PAE, can be achieved at 90.5%. Two patients had pelvic vascular obliteration by atherosclerosis, so we did unilateral PAE.
- PAE is a relatively safe method except for possible mis-embolization, there is no expected major complication. In recent meta-analysis found that major complication rate was 0.3% due to nontarget embolization, and the most common minor complications were dysuria (17.0%) and transient increased urinary frequency (11.6%) [34]. In this study, adverse events were summarized in Table 3. There were no major complications (>3 Clavian complications) and nontarget embolization. Nine patients had minor complications. Four patients (19%) had a urinary tract infection, and they were controlled by 2 weeks of antibiotics. Two patients (9.5%) felt burning sense of the perineum and they were controlled by NSAID only. Three patients developed urge incontinence after removed catheter. They needed 2 to 3 diapers per day immediate after removed catheter for managing urge incontinence, but this symptom was resolved by conservative care with anticholinergics.
- Limitations of this study include the single-center, retrospective fashion, which may decrease the quality of evidence. Second, the sample size of patients was small and there was a selection of bios. This small sample size probably influenced the results of this study to obtain relatively better results compared to other studies. Additionally, we reported the results of 1-year follow-up, but the follow-up period was not sufficient. Despite the small sample size and insufficient follow-up period, reduction in prostate size and improvement of LUTS were confirmed in patients with an enlarged prostate greater than 100 mL at risk of surgery, and the clinical usefulness of PAE was confirmed. In the future, additional studies are needed on the efficacy of PAE in the enlarged prostatic hyperplasia of the median lobe and small benign prostate hyperplasia patients.
- In conclusion, PAE could be a secondary option for treating large BPH (over 100 mL) and urinary retention or gross hematuria associated with BPH in men unfit for surgery. Proper patient selection through evaluation is especially important to ensure clinical success.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MISIT) (No. 2018R1C1B5040967).
-
Author contributions
All the work was done by SK.
Article information
Fig. 1.Comparisons of the following parameters from the preoperative assessment through 1, 3, 6, and 12 months postoperatively: (A) mean International Prostate Symptom Score (IPSS); (B) mean quality of life (QoL) score; (C) mean peak urine flow rate (Qmax); (D) mean post-void residual (PVR) urine volume; (E) mean prostate-specific antigen (PSA) level; and (F) mean prostate volume. PAE, prostate artery embolization.

Table 1.Baseline clinical characteristics of participants prior to PAE
Table 2.Summary of the mean changes from baseline at 1, 3, 6, and 12 months
Table 3.Adverse events (n=21)
| Minor complication | No. (%) | Managements |
|---|---|---|
| Urinary tract infection | 4 (19.0) | 2 wk antibiotics |
| Urge incontinence | 3 (14.2) | Anti-cholinergic medication |
| Pelvic pain | 2 (9.5) | NSAID with conservative care |
- 1. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol 2005;7(Suppl 9):S3–14.
- 2. Langan RC. Benign prostatic hyperplasia. Prim Care 2019;46:223–32.ArticlePubMed
- 3. Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: what you need to know. J Urol 2006;175(3 Pt 2):S19–24.ArticlePubMed
- 4. Woodard TJ, Manigault KR, McBurrows NN, Wray TL, Woodard LM. Management of benign prostatic hyperplasia in older adults. Consult Pharm 2016;31:412–24.ArticlePubMed
- 5. Wilhelm K. Benign prostatic hyperplasia: possibilities of microwave thermotherapy. Urologe A 2018;57:1366–9.PubMed
- 6. Te AE. Recent advances in prostatectomy for benign prostatic hyperplasia. F1000Res 2019;8(F1000 Faculty Rev):1528.ArticlePDF
- 7. Sievert KD, Schonthaler M, Berges R, Toomey P, Drager D, Herlemann A, et al. Minimally invasive prostatic urethral lift (PUL) efficacious in TURP candidates: a multicenter German evaluation after 2 years. World J Urol 2019;37:1353–60.ArticlePubMedPDF
- 8. Kably I, Acharya V, Richardson AJ, Bhatia S. Prostatic artery embolization in refractory hematuria of prostatic origin. Tech Vasc Interv Radiol 2020;23:100694.ArticlePubMed
- 9. Carnevale FC, Antunes AA, da Motta Leal Filho JM, de Oliveira Cerri LM, Baroni RH, Marcelino AS, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovasc Intervent Radiol 2010;33:355–61.ArticlePubMedPDF
- 10. Lee HS. Ethical issues in clinical research and publication. Kosin Med J 2022;37:278–82.ArticlePDF
- 11. Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol 2018;200:612-9.Article
- 12. Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118–40.ArticlePubMed
- 13. Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomised clinical trial. Eur Urol 2008;53:160–6.ArticlePubMed
- 14. Shah HN, Mahajan AP, Hegde SS, Bansal MB. Peri-operative complications of holmium laser enucleation of the prostate: experience in the first 280 patients, and a review of literature. BJU Int 2007;100:94–101.ArticlePubMed
- 15. Kurbatov D, Russo GI, Lepetukhin A, Dubsky S, Sitkin I, Morgia G, et al. Prostatic artery embolization for prostate volume greater than 80 cm3: results from a single-center prospective study. Urology 2014;84:400–4.ArticlePubMed
- 16. de Assis AM, Moreira AM, de Paula Rodrigues VC, Yoshinaga EM, Antunes AA, Harward SH, et al. Prostatic artery embolization for treatment of benign prostatic hyperplasia in patients with prostates > 90 g: a prospective single-center study. J Vasc Interv Radiol 2015;26:87–93.ArticlePubMed
- 17. Wang MQ, Guo LP, Zhang GD, Yuan K, Li K, Duan F, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (>80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urol 2015;15:33.ArticlePubMedPMCPDF
- 18. Bhatia S, Sinha VK, Harward S, Gomez C, Kava BR, Parekh DJ. Prostate artery embolization in patients with prostate volumes of 80 mL or more: a single-institution retrospective experience of 93 patients. J Vasc Interv Radiol 2018;29:1392–8.ArticlePubMed
- 19. Bhatia S, Sinha VK, Kava BR, Gomez C, Harward S, Punnen S, et al. Efficacy of prostatic artery embolization for catheter-dependent patients with large prostate sizes and high comorbidity scores. J Vasc Interv Radiol 2018;29:78–84.ArticlePubMed
- 20. Feng S, Tian Y, Liu W, Li Z, Deng T, Li H, et al. Prostatic arterial embolization treating moderate-to-severe lower urinary tract symptoms related to benign prostate hyperplasia: a meta-analysis. Cardiovasc Intervent Radiol 2017;40:22–32.ArticlePubMedPDF
- 21. Gao YA, Huang Y, Zhang R, Yang YD, Zhang Q, Hou M, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate: a prospective, randomized, and controlled clinical trial. Radiology 2014;270:920–8.ArticlePubMed
- 22. Lebdai S, Delongchamps NB, Sapoval M, Robert G, Amouyal G, Thiounn N, et al. Early results and complications of prostatic arterial embolization for benign prostatic hyperplasia. World J Urol 2016;34:625–32.ArticlePubMedPDF
- 23. Pisco JM, Rio Tinto H, Campos Pinheiro L, Bilhim T, Duarte M, Fernandes L, et al. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol 2013;23:2561–72.ArticlePubMedPDF
- 24. Camara-Lopes G, Mattedi R, Antunes AA, Carnevale FC, Cerri GG, Srougi M, et al. The histology of prostate tissue following prostatic artery embolization for the treatment of benign prostatic hyperplasia. Int Braz J Urol 2013;39:222–7.ArticlePubMed
- 25. Bilhim T, Pisco JM, Rio Tinto H, Fernandes L, Pinheiro LC, Furtado A, et al. Prostatic arterial supply: anatomic and imaging findings relevant for selective arterial embolization. J Vasc Interv Radiol 2012;23:1403–15.ArticlePubMed
- 26. Bilhim T, Pisco JM, Furtado A, Casal D, Pais D, Pinheiro LC, et al. Prostatic arterial supply: demonstration by multirow detector angio CT and catheter angiography. Eur Radiol 2011;21:1119–26.ArticlePubMedPDF
- 27. A Pereira J, Bilhim T, Duarte M, Rio Tinto H, Fernandes L, Martins Pisco J. Patient selection and counseling before prostatic arterial embolization. Tech Vasc Interv Radiol 2012;15:270–5.ArticlePubMed
- 28. Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, et al. Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. J Vasc Interv Radiol 2016;27:1115–22.ArticlePubMed
- 29. Isaacson AJ, Raynor MC, Yu H, Burke CT. Prostatic artery embolization using embosphere microspheres for prostates measuring 80-150 cm(3): early results from a US trial. J Vasc Interv Radiol 2016;27:709–14.ArticlePubMed
- 30. Maclean D, Maher B, Harris M, Dyer J, Modi S, Hacking N, et al. Planning prostate artery embolisation: is it essential to perform a pre-procedural CTA? Cardiovasc Intervent Radiol 2018;41:628–32.ArticlePubMedPDF
- 31. Abt D, Hechelhammer L, Mullhaupt G, Markart S, Gusewell S, Kessler TM, et al. Comparison of prostatic artery embolization (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ 2018;361:k2338.ArticlePubMedPMC
- 32. Chiaradia M, Radaelli A, Campeggi A, Bouanane M, De La Taille A, Kobeiter H. Automatic three-dimensional detection of prostatic arteries using cone-beam CT during prostatic arterial embolization. J Vasc Interv Radiol 2015;26:413–7.ArticlePubMed
- 33. Bilhim T, Pisco J, Rio Tinto H, Fernandes L, Campos Pinheiro L, Duarte M, et al. Unilateral versus bilateral prostatic arterial embolization for lower urinary tract symptoms in patients with prostate enlargement. Cardiovasc Intervent Radiol 2013;36:403–11.ArticlePubMedPDF
- 34. Malling B, Roder MA, Brasso K, Forman J, Taudorf M, Lonn L. Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Radiol 2019;29:287–98.ArticlePubMedPDF
References
Figure & Data
References
Citations
Citations to this article as recorded by 


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite