Articles
- Page Path
- HOME > Kosin Med J > Volume 38(4); 2023 > Article
-
Case report
Drug-induced immune-mediated thrombocytopenia due to bevacizumab-FOLFOX therapy: a case report -
Minna Kim*
 , Jong Hoon Lee*
, Jong Hoon Lee* , Jong Yoon Lee
, Jong Yoon Lee
-
Kosin Medical Journal 2023;38(4):300-306.
DOI: https://doi.org/10.7180/kmj.23.121
Published online: June 12, 2023
Division of Gastroenterology, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- Corresponding Author: Jong Yoon Lee, MD, MS Division of Gastroenterology, Department of Internal Medicine, Dong-A University College of Medicine, 32 Daesingongwon-ro, Seo-gu, Busan 49201, Korea Tel: +82-51-240-5042 Fax: +82-51-242-5852 E-mail: ljyhateo@gmail.com
- *These authors contributed equally to this work as first authors.
Copyright © 2023 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 825 Views
- 22 Download
Abstract
- Drug-induced immune thrombocytopenia (DITP) is a very rare disease, with an estimated annual incidence of 10 cases per million. Oxaliplatin and irinotecan are widely used as chemotherapy for high-risk stage II and III colorectal cancer, and DITP has been reported to occur in patients using those agents. To treat unresectable metastatic colorectal cancer, bevacizumab is used in combination with oxaliplatin or irinotecan, and there have been a few reports of DITP cases in patients receiving that regimen. In this report, we describe a 68-year-old male patient with metastatic colon cancer (KRAS mutant type) to the liver and lung who developed acute immune-mediated thrombocytopenia due to bevacizumab-FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) therapy. During treatment, he showed purpura in his lower extremities on 21st cycle day 2. Lab work revealed a platelet count of less than 2,000/μL, reflecting a decrease from 135,000/μL at the start of the cycle 1 day prior. He did not have any other types of cytopenia or significant changes in laboratory findings. We diagnosed DITP due to bevacizumab-FOLFOX, and the patient did not show isolated thrombocytopenia after switching to Ziv-aflibercept-FOLFIRI (5-fluorouracil, leucovorin, and irinotecan).
- Drug-induced thrombocytopenia occurs when certain medications destroy platelets or hinder the body's platelet production. Two distinct types of drug-induced thrombocytopenia exist: immune and nonimmune. Drug-induced nonimmune thrombocytopenia arises when a medication hampers the bone marrow's ability to produce an adequate quantity of platelets. On the other hand, drug-induced immune thrombocytopenia (DITP), a medication prompts the body to generate antibodies that target and destroy platelets. DITP is a very rare disease, with an estimated annual incidence of 10 cases per million people [1]. However, this number may be underestimated as DITP is not always recognized as a cause of thrombocytopenia and can be misdiagnosed or diagnosed with delay [2]. Although thrombocytopenia is a common side effect of chemotherapy caused by myelosuppression affecting all blood cell types, DITP is characterized by isolated thrombocytopenia instead of bicytopenia or pancytopenia. Anticancer drugs such as fludarabine, dactinomycin, cisplatin, oxaliplatin, and irinotecan have been reported as rare causes of DITP [3-5]. In the treatment of high-risk stage II and III colorectal cancer, oxaliplatin and irinotecan are commonly used in adjuvant chemotherapy regimens and have been reported to cause DITP [6-9]. However, for patients with unresectable metastatic colorectal cancer, targeted therapies are often combined with oxaliplatin or irinotecan, and reports of DITP in patients receiving such combination treatments are rare.
- The authors report a rare case of DITP in a patient receiving bevacizumab-FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) therapy as palliative treatment for unresectable metastatic colorectal cancer. In this report, the authors aim to provide a literature review on DITP and highlight the importance of recognizing and managing this rare complication in cancer patients.
Introduction
- Ethics statements: The study protocol was reviewed and approved by the Institutional Review Board of the Dong-A University College of Medicine (DAUHIRB-23-056). Informed consent for publication of clinical data was obtained from the case patient.
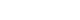
- A 68-year-old male was admitted for palliative chemotherapy for metastatic colorectal cancer. He had undergone self-expandable metal stent insertion due to colorectal cancer-related obstruction (Fig. 1), followed by laparoscopic anterior resection and metastasectomy of the liver and lung. However, residual cancer was found in the resected liver metastasis, rendering further surgery impossible (Fig. 2). Therefore, the patient was treated with systemic chemotherapy using FOLFOX in combination with bevacizumab, a vascular endothelial growth factor (VEGF) monoclonal antibody agent, as the patient was found to be epidermal growth factor receptor (EGFR)-positive but KRAS-mutant by immunohistochemistry. After 20 cycles of chemotherapy, the patient did not report any significant side effects, and based on RECIST criteria, was found to have stable disease on computed tomography (CT) scans conducted every three cycles.
- Therefore, the patient started receiving the 21st cycle of bevacizumab-FOLFOX. During the physical examination, no specific findings were observed, and vital signs were stable with a blood pressure of 120/80 mmHg, a body temperature of 36.4 ℃, a pulse rate of 89 beats/min, and a respiratory rate of 16 breaths/min. Laboratory findings showed white blood cells at 5,850/μL, hemoglobin at 14.2 g/dL, platelets at 135,000/μL, creatinine at 0.72 mg/dL, C-reactive protein at 0.09 mg/dL, prothrombin time at 12 seconds, and international normalized ratio at 1.08. Other findings were aspartate aminotransferase at 24 U/L, alanine aminotransferase at 5 U/L, albumin at 4.1 g/dL, total bilirubin at 0.5 mg/dL, serum sodium at 135 mmol/L, and serum potassium at 4.7 mmol/L, all within normal limits. The polymerase chain reaction for coronavirus disease 2019 was negative. Chemotherapy was started on the 2nd day of hospitalization, with bevacizumab at a dose of 5 mg/kg intravenously for a total of 270 mg, and oxaliplatin at a dose of 85 mg/m2 for a total of 130 mg, without any specific changes. On the 3rd day of hospitalization, which was the 2nd day of chemotherapy, purpura was observed in both lower extremities (Fig. 3A, 3B). In the blood test, platelets were measured to be less than 2,000/μL, and the same value was confirmed on retesting, indicating a rapid decrease in platelets. Depending on the Common Terminology Criteria for Adverse Events, it can be classified as grade 4, an adverse event that seriously interferes with life. Although hemoglobin decreased to 11.5 g/dL, there were no other bleeding signs except for purpura on the limbs. There were no significant changes in other blood cell disorders, prothrombin, aminotransferase, bilirubin, creatinine, lactate dehydrogenase, or electrolytes. Additionally, no abnormalities were observed in peripheral blood smears, including fragmented red blood cells, left shift, immature cells, or platelet aggregation, and coagulation tests, including D-dimer, were normal. Anti-platelet antibodies and platelet-associated antibodies were negative. Chemotherapy was immediately discontinued, and 320 mL of platelets in eight units were transfused. After the transfusion, the platelet count rose to 81,000/μL, and without additional transfusion, the patient was discharged after further observation with the platelet count maintaining at 67,000/μL on the 6th day of hospitalization and showed improvement in purpura on the limbs with no other bleeding signs (Fig. 3C). One week later, the platelet count was confirmed to be 212,000/μL in an outpatient visit (Fig. 4). Since the patient's platelets had recovered, no cytopenia was observed except for platelets, and no abnormalities were detected in the peripheral blood smear. Therefore, it was decided, in consultation with the hematologist, not to proceed with additional invasive tests such as a bone marrow biopsy for the patient.
- After the last chemotherapy, a CT scan was performed, and the patient was confirmed to have progressive disease according to the response evaluation criteria in solid tumors (Fig. 5). After changing to Ziv-aflibercept-FOLFIRI (5-fluorouracil, leucovorin, and irinotecan), the patient underwent nine cycles of chemotherapy without a decrease in platelet count.
Case
- For the treatment of unresectable metastatic colorectal cancer, combination chemotherapy based on FOLFOX containing oxaliplatin or FOLFIRI containing irinotecan is established as first-line treatment, with the addition of bevacizumab, a monoclonal antibody targeted therapy for VEGF, or cetuximab, a monoclonal antibody targeted therapy for EGFR [10]. FOLFOX is widely used for the treatment of high-risk stage 2 and stage 3 colorectal cancer [3]. Compared to cetuximab, which is effective only for left-sided colon cancer with positive EGFR and wild-type KRAS and NRAS, bevacizumab is relatively more applicable in the palliative treatment of metastatic colorectal cancer [11].
- Oxaliplatin is commonly associated with peripheral neuropathy and asymptomatic pancytopenia, but there have also been reports of hypersensitivity reactions and isolated cases of thrombocytopenia [3,12]. There are three mechanisms for thrombocytopenia caused by oxaliplatin. The first mechanism is bone marrow suppression. A significant proportion of thrombocytopenia can be explained by bone marrow suppression. A study showed that thrombocytopenia was observed in 19% of patients treated with 5-fluorouracil/leucovorin alone, but in 77% of patients treated with oxaliplatin in combination, most of which could be explained by bone marrow suppression accompanied by anemia and leukopenia [3]. Although the mechanism is not clearly understood, it is believed that oxaliplatin induces apoptosis in megakaryocytes more than other drugs, leading to thrombocytopenia. This is accompanied by other blood cell decreases, occurs around 10 days after administration, is dose-dependent, and gradually appears, making it predictable. This is a major mechanism for causing thrombocytopenia [6]. The second mechanism is splenic sequestration caused by sinusoidal injury. Oxaliplatin is believed to cause thrombocytopenia through sinusoidal injury, which leads to secondary portal hypertension, splenomegaly, and sequestration of platelets [13,14]. According to a study conducted by M.D. Anderson in the United States, splenomegaly caused by oxaliplatin-based therapy can be measured using the splenic index. When comparing patients who received FOLFOX and those who received 5-fluorouracil/leucovorin, an increase in splenic index was observed in 45.7% and 16.3% of patients, respectively. Of those who developed splenomegaly, 28% experienced thrombocytopenia, while only 5% of patients without splenomegaly had this complication [15]. The third mechanism is immune-mediated thrombocytopenia. Although the exact mechanism of platelet destruction is not fully understood, it can be associated with weakly binding antibodies to platelet membrane antigens. Drug-dependent anti-platelet antibodies to specific drugs such as oxaliplatin can also cause platelet destruction and thrombocytopenia through interaction with antigens. This immune-mediated thrombocytopenia caused by these drugs usually occurs within 2 weeks of exposure, with some cases appearing rapidly within a few hours. Platelet counts often drop to less than 20,000/μL and are unpredictable [16]. However, considering a rare case report in which myelodysplastic syndrome was diagnosed through a bone marrow biopsy in a gastric cancer patient experiencing gradual thrombocytopenia using FOLFOX, it would be necessary to consult with a hematologist for evaluation if hematologic malignancies as the underlying cause of thrombocytopenia cannot be ruled out [17].
- In this case, the patient exhibited isolated thrombocytopenia without reductions in other blood cells. The rapid onset of a sudden decrease, rather than a gradual decline, suggests the possibility of immune-mediated thrombocytopenia rather than bone marrow suppression. Abdominal CT did not reveal splenomegaly, and there were no significant decreases in other blood cells apart from platelets. Additionally, no abnormal findings were observed in the peripheral blood smear, supporting the clinical plausibility of a diagnosis of DITP. The authors attempted to confirm the presence of drug-dependent anti-platelet antibodies using flow cytometry, but due to limitations in hospital resources, this was not feasible. While the presence of drug-dependent anti-platelet antibodies may support the diagnosis, it is not a prerequisite. Clinical diagnosis of DITP can be made by observing whether platelet count recovers after discontinuing the drug. A negative result for drug-dependent anti-platelet antibodies does not exclude the diagnosis [18]. Also, it can be difficult for most medical institutions to confirm drug-dependent anti-platelet antibodies for a specific drug, and the process of obtaining test results can take several days or longer. Therefore, immediate diagnosis and drug discontinuation should be determined through clinical diagnosis. However, it may be difficult to determine which drug is causing DITP through clinical diagnosis alone. Bevacizumab, administered along with FOLFOX, can cause fatal side effects such as hypertension, proteinuria, thrombosis, and gastrointestinal perforation [19]. Compared to reports of oxaliplatin, a component of FOLFOX, being a causative agent of immune-mediated thrombocytopenia in some studies, reports of immune-mediated thrombocytopenia caused by bevacizumab are rare [7-9,20]. In this case, after the last administration of bevacizumab-FOLFOX, the patient was found to have progressive disease on follow-up abdominal CT and had to switch to another chemotherapy regimen, Ziv-aflibercept FOLFIRI, after which there was no recurrence of thrombocytopenia. Therefore, the cause of thrombocytopenia, in this case, was diagnosed as DITP caused by bevacizumab-FOLFOX. Based on previous studies, oxaliplatin is the most commonly reported drug among the administered drugs, making it the most likely cause, but the possibility of bevacizumab cannot be ruled out. Although the exact cause of the DITP was not determined by identifying drug-dependent platelet antibodies, it is important to make a clinical judgment in the diagnosis of DITP. The fact that no recurrence was observed after changing the anticancer therapy and understanding the mechanism of DITP is significant.
- The authors report a rare case of DITP that occurred during palliative chemotherapy for unresectable metastatic colon cancer, along with a literature review.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: JYL. Data curation: JYL. Formal analysis: JYL. Investigation: MK, JHL, JYL. Methodology: MK, JHL, JYL. Project administration: MK, JHL, JYL. Supervision: JHL, JYL. Validation: MK, JHL, JYL. Visualization: MK, JHL, JYL. Writing - original draft: MK, JYL. Writing - review & editing: MK, JHL, JYL.
Article information




- 1. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med 2007;357:580–7.ArticlePubMed
- 2. George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program 2009;153–8.ArticlePubMedPDF
- 3. Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51.ArticlePubMed
- 4. Arnold DM, Kukaswadia S, Nazi I, Esmail A, Dewar L, Smith JW, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost 2013;11:169–76.ArticlePubMedPMCPDF
- 5. Mitta A, Curtis BR, Reese JA, George JN. Drug-induced thrombocytopenia: 2019 update of clinical and laboratory data. Am J Hematol 2019;94:E76–8.ArticlePubMed
- 6. Jardim DL, Rodrigues CA, Novis YA, Rocha VG, Hoff PM. Oxaliplatin-related thrombocytopenia. Ann Oncol 2012;23:1937–42.ArticlePubMed
- 7. Suh SE, Jang MJ, Chong SY, Aster RH, Curtis BR, Oh D. A case of oxaliplatin-induced immune-mediated thrombocytopenia. Blood Res 2014;49:61–4.ArticlePubMedPMC
- 8. Tam EL, Draksharam PL, Park JA, Sidhu GS. Acute immune-mediated thrombocytopenia due to oxaliplatin and irinotecan therapy. Case Rep Oncol Med 2019;2019:4314797.ArticlePubMedPMCPDF
- 9. Stack A, Khanal R, Denlinger CS. Oxaliplatin-induced immune thrombocytopenia: a case report and literature review. Clin Colorectal Cancer 2021;20:e1–4.ArticlePubMed
- 10. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:329–59.PubMed
- 11. Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563–72.ArticlePubMed
- 12. Mori Y, Nishimura T, Kitano T, Yoshimura K, Matsumoto S, Kanai M, et al. Oxaliplatin-free interval as a risk factor for hypersensitivity reaction among colorectal cancer patients treated with FOLFOX. Oncology 2010;79:136–43.ArticlePubMedPDF
- 13. Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010;28:2549–55.ArticlePubMed
- 14. Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer 2009;8:225–30.ArticlePubMed
- 15. Angitapalli R, Litwin AM, Kumar PR, Nasser E, Lombardo J, Mashtare T, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II-III colorectal cancer. Oncology 2009;76:363–8.ArticlePubMedPDF
- 16. George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med 1998;129:886–90.ArticlePubMed
- 17. Seo KI, Kim SE, Park MI, Park SJ, Moon W, Han YJ. A case of therapy-related myelodysplastic syndrome after FOLFOX4 chemotherapy in advanced gastric cancer. J Dig Cancer Rep 2016;4:43–5.
- 18. Bougie D, Aster R. Immune thrombocytopenia resulting from sensitivity to metabolites of naproxen and acetaminophen. Blood 2001;97:3846–50.ArticlePubMedPDF
- 19. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 2021;325:669–85.ArticlePubMed
- 20. Kumar J, Bhargava M, Aggarwal S. Bevacizumab-induced reversible thrombocytopenia in a patient with adenocarcinoma of colon: rare adverse effect of bevacizumab. Case Rep Oncol Med 2012;2012:695430.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite