Clinical efficacy and safety of autologous serum intramuscular injection in patients with mild-to-moderate atopic dermatitis: a prospective, open-label, uncontrolled study
Article information
Abstract
Background
Autologous blood therapy (ABT) has been used to treat atopic dermatitis (AD) for over a century, even though evidence supporting its efficacy is lacking. We aimed to investigate the effectiveness and safety of autologous serum intramuscular injection (ASIM), which is a modified form of ABT, in treating mild-to-moderate AD.
Methods
This study was a 12-week, open-label, prospective, uncontrolled trial. Following a 4-week run-in period, 22 out of 25 screened patients received ASIM once a week for 4 weeks in conjunction with standard treatment. The primary outcome measure was the Eczema Area and Severity Index (EASI), while the secondary outcomes included the Scoring Atopic Dermatitis (SCORAD) score, Dermatologic Life Quality Index (DLQI), and patient ratings of pruritus, sleep difficulty, disease status, and treatment effectiveness. Safety parameters were also assessed.
Results
EASI scores showed a non-statistically significant trend toward improvement during ASIM intervention. Patients with at least a 50% improvement in the EASI score at 4 weeks were older and had lower peripheral eosinophil counts (p<0.05). Secondary endpoints, including the SCORAD score, pruritus, sleep difficulty, and DLQI, demonstrated statistically significant improvements at week 4 compared to baseline (p<0.05). No significant adverse reactions were observed.
Conclusions
This pioneering study suggests that repeated ASIM may improve the clinical symptoms of mild-to-moderate AD, particularly in terms of pruritus and overall quality of life. However, further research with a larger sample size is required to establish the clinical significance of these findings.
Introduction
Atopic dermatitis (AD) is a chronic, recurring inflammatory skin disease that is characterized by skin dryness, pruritus, and rashes [1]. Over the past few decades, the prevalence and incidence of AD have increased, with reported prevalence rates of 15%–20% and 10% in children and adults, respectively [1,2]. Current conventional medical treatments for AD, such as topical corticosteroids and/or topical calcineurin inhibitors, mainly aim to alleviate symptoms and have incomplete clinical efficacy, thereby causing frequent disappointment to both patients and physicians. Several conventional systemic immunosuppressants such as glucocorticoids, cyclosporine, or methotrexate, have been used in the treatment of AD, but these medications can cause serious side effects. Recently, substantial progress has been made in the development of Th2-targeted therapy, including dupilumab and Janus kinase (JAK) inhibitors, however, such therapies have limited clinical use because of their high cost and side effects. Therefore, the use of new therapeutic modalities should be explored for patients with AD, particularly in those who respond insufficiently to standard medical treatments.
Autologous blood therapy (ABT), which is also known as autohemotherapy, involves extracting small amounts of blood from a patient and then reinjecting intramuscularly. It is a type of alternative medicine that has been used for the treatment of allergic disease, including urticaria and AD, in North America, Europe, and Japan for over a century [3-5]. Data on the therapeutic effect of ABT for AD are rare. One randomized, double-blind, placebo-controlled study has demonstrated the favorable clinical efficacy in patients with AD [6]. Autologous serum intramuscular injection (ASIM) is a modified form of ABT in which only serum isolated from the blood is intramuscularly injected into the patient. It is considered superior to ABT because circulating autoreactive factors (including immunoglobulin G autoantibodies) are present in the serum rather than in the blood cell compartment and is less painful than ABT [7,8]. Cho et al. [9] suggested that autologous plasma therapy is effective in patients with AD, particularly the plasma fraction of the blood components is linked to its therapeutic effect. Therefore, we hypothesized that repeated ASIM treatments could induce clinical improvement in patients with AD. In this study, we aimed to evaluate the effectiveness and safety of ASIM in patients with mild-to-moderate AD who were concurrently receiving standard medical therapy.
Methods
Ethical statements: This study underwent review and received approval from the Institutional Review Board of Kosin University Gospel Hospital (approval no. 2018-05-018). Written informed consent was obtained.
1. Study design and patients
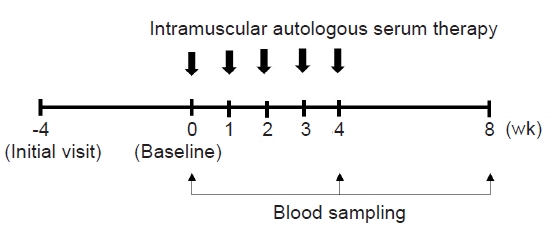
This was a 12-week, prospective, open-label, uncontrolled study that was conducted between December 2019 and May 2021 at an outpatient clinic. The study comprised a 4-week screening and washout phase, followed by a 4-week intervention phase, and concluded with a 4-week follow-up period (Fig. 1). Topical corticosteroids and oral antihistamines remained standard treatments throughout the study period. Systemic corticosteroids were administered to the patients as emergency therapy to alleviate intolerable symptoms of AD, at the discretion of investigators.
Eligible patients were adolescent and adult individuals (≥13 years old) with mild-to-moderate AD who met the diagnostic criteria for AD proposed by the American Dermatological Society. Mild-to-moderate AD was defined as AD that was inadequately controlled with topical corticosteroids and oral antihistamines after 1 month of treatment, and had a Scoring Atopic Dermatitis (SCORAD) score <50. Patients with concurrent other active skin disease, individuals receiving or expected to require treatment with systemic immunomodulatory medication (such as corticosteroids, methotrexate, and cyclosporine) or ultraviolet light at the time of initial screening and baseline, pregnant patients, those with alcoholism, and individuals with severe systemic disease were excluded. Patients were assessed during their routine clinic visit, and those who agreed to participate were enrolled after obtaining their written informed consent [10,11].
2. ASIM therapy
Upon the final determination of study eligibility at the baseline visit (week 0), the patients received five weekly ASIM over a 4-week duration (from baseline to week 4) (Fig. 1). For ASIM, 5–10 mL of blood was drawn using a sterile disposable syringe from the patient’s antecubital vein and then centrifuged at 3,000 rpm for 10 minutes at room temperature. Only 2.5 mL of serum is collected from centrifuged blood and immediately injected intramuscularly into the gluteal muscle using a 24-gauge needle.
3. Outcome measures
The primary endpoint was to assess the change in clinical severity score utilizing the Eczema Area and Severity Index (EASI) from baseline to week 8 [12]. Additionally, secondary outcome measures including the SCORAD, Investigator's Global Assessment (IGA), Dermatologic Life Quality Index (DLQI) scores, and patient assessments of itchiness and sleep difficulty, each measured on a 10 cm visual analogue scale (VAS), were assessed [13-16]. Perceived overall effectiveness (a 7-point scale ranging from very much worse to very much improved) and disease status (a 6-point scale ranging from clear to very severe symptoms) were also assessed. The primary and secondary outcome measures, along with medication usage, were evaluated at baseline, weeks 2 and 4, and at the end of the follow-up period, week 8. The EASI, SCORAD, and IGA scores were measured by the same dermatologist. Safety was evaluated by assessing the occurrence of adverse events, clinical laboratory tests, vital signs, and physical examinations.
4. Assessment of serum cytokine levels
To evaluate their possible association of ASIM with AD, the serum concentrations of interleukins (ILs) 13 and 31, and interferon-gamma (IFN-γ) were measured using commercially available enzyme-linked immunosorbent assay kits (Abcam) following the manufacturer’s guidelines.
5. Statistical analysis
Data were expressed as mean±standard deviation or as number (%). The statistical significance of the differences in values pre- and post-ASIM was evaluated using the paired t-test or Wilcoxon signed-rank test for continuous variables, and the chi-square test for categorical variables. Statistical analyses were conducted utilizing SPSS software version 25.0 (IBM Corp.). A p-value of less than 0.05 was considered statistically significant.
Results
1. Study patients
Among the initial 25 patients screened, 23 fulfilled the inclusion criteria received ASIM once a week for 4 weeks. However, one patient was lost to follow-up owing to the coronavirus disease 2019 (COVID-19) pandemic; therefore, 22 patients successfully completed the interventions. Table 1 summarizes the characteristics of the patients. The mean age of the patients was 25.8±6.4 years, and males made up 36.4% of the patient population. The mean disease duration of AD and persistent treatment duration prior to enrollment were 159.91±89.24 months and 50.27±38.97 months, respectively. Four patients (18.2%) tested positive on the autologous serum skin test.
2. Clinical efficacy
In terms of the primary endpoint, the EASI score demonstrated a tendency to improve during ASIM intervention, although the difference was not statistically significant (Fig. 2). At week 4, 16 patients (69.6%) demonstrated improvement in the EASI score, with a mean improvement of –51.5% compared to the baseline score. Improvements of at least 50% in the EASI score (EASI-50) at weeks 4 and 8 were reported in 40.9% and 18.2% of patients, respectively (Table 2). Patients who responded to the EASI-50 at week 4 were older (29.78±5.09 years vs. 22.69±5.72 years), and lower peripheral eosinophil counts (155.6±114.7/mm3 vs. 310.6±197.3/mm3) than the non-responders (p=0.004, p=0.009, respectively).
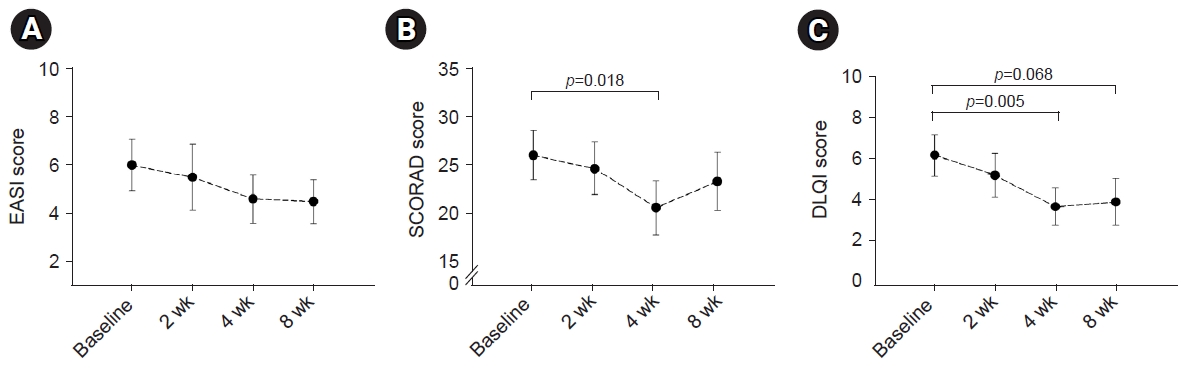
Changes in the EASI score (A), SCORAD score (B), and DLQI score (C) during the intervention. The EASI score demonstrated a non-significant tendency to improve during the ASIM intervention, while the SCORAD and DLQI scores showed significant improvements. Data are presented as the mean±standard error. The statistical significance of differences was assessed by the t-test. EASI, Eczema Area and Severity Index; SCORAD, Scoring Atopic Dermatitis; DLQI, Dermatology Life Quality Index; ASIM, autologous serum intramuscular injection.
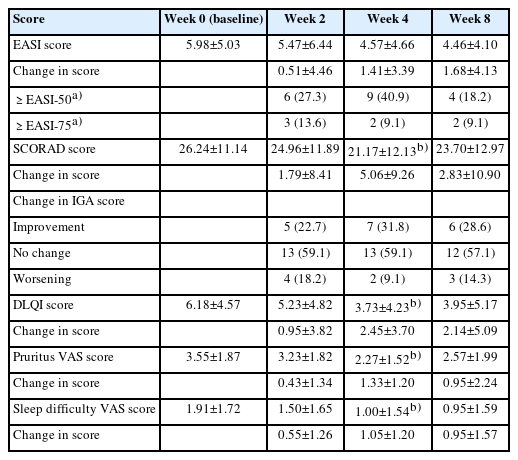
Changes in primary and secondary clinical outcome measures during autologous serum intramuscular injection
In terms of the secondary endpoint, the SCORAD scores decreased at week 4 of intervention and at the 8-week follow-up visit after intervention. Furthermore, a statistically significant improvement was observed at week 4 (p=0.018) (Fig. 2). Similarly, the DLQI and VAS scores for pruritus and sleep difficulty demonstrated statistically significant improvements at week 4 compared to the baseline scores. The IGA scores demonstrated improvement in seven patients (31.8%) after 4 weeks of treatment, and this pattern was maintained for 8 weeks following treatment (Table 2). There were no statistically significant changes noted in the utilization of antihistamines or topical corticosteroids, although a slight tendency towards decreased usage was observed after 8 weeks of intervention (data not shown).
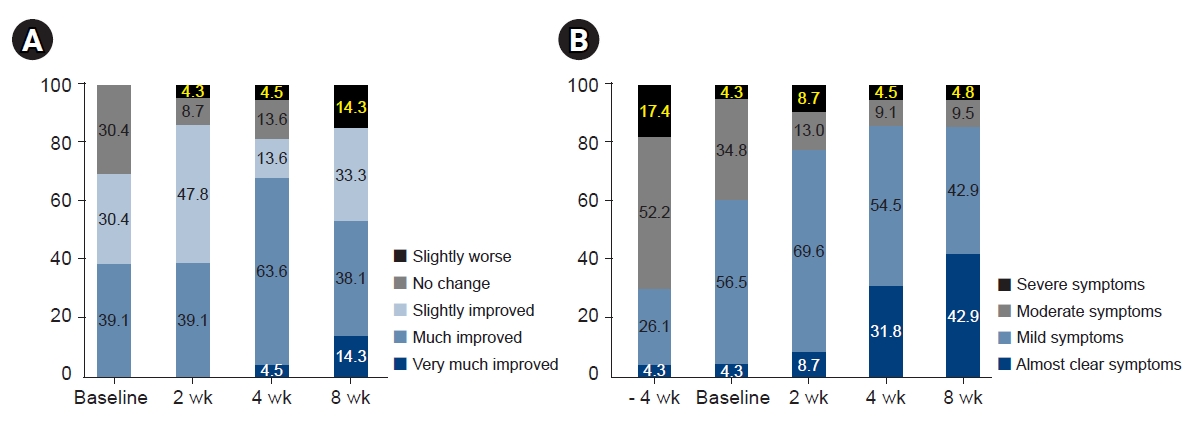
In the evaluation of patients’ overall treatment effect, the proportion of patients reporting slightly improvements began to increase from week 2, and 68.1% of patients reported that they experienced much or very much improvements. Although the proportion of patients demonstrating improvement remained constant at week 8, 14.3% of patients responded that their condition had slightly worsened (Fig. 3). In the evaluation of patients' perceived disease state, the proportion of patients reporting mild/almost clear symptoms began to increase from week 2. By week 8, 42.9% of patients reported that they had almost clear symptoms. However, no notable difference was found in the proportion of patients who perceived that their symptoms as severe (Fig. 3).
3. Safety
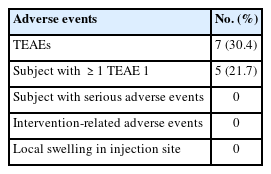
Treatment-emergent adverse events were reported in 21.7% of patients (Table 3). These adverse events included headache, diarrhea, chest discomfort, acne, general pain, ear infection, and nasal congestion. The patients did not report any adverse reactions related to the injection, such as local pain, swelling, and ecchymosis at the injection site. There were no reports of serious adverse events, and no patients discontinued ASIM.
4. Changes in serum IL-13, IL-31, and IFN-γ levels
There were no changes in the level of IL-13. The level of IL-31 demonstrated a tendency to slightly increase during ASIM and follow-up period, whereas the level of IFN-γ decreased during the treatment and increased at the follow-up period, although no statistically significant changes were observed (Fig. 4).
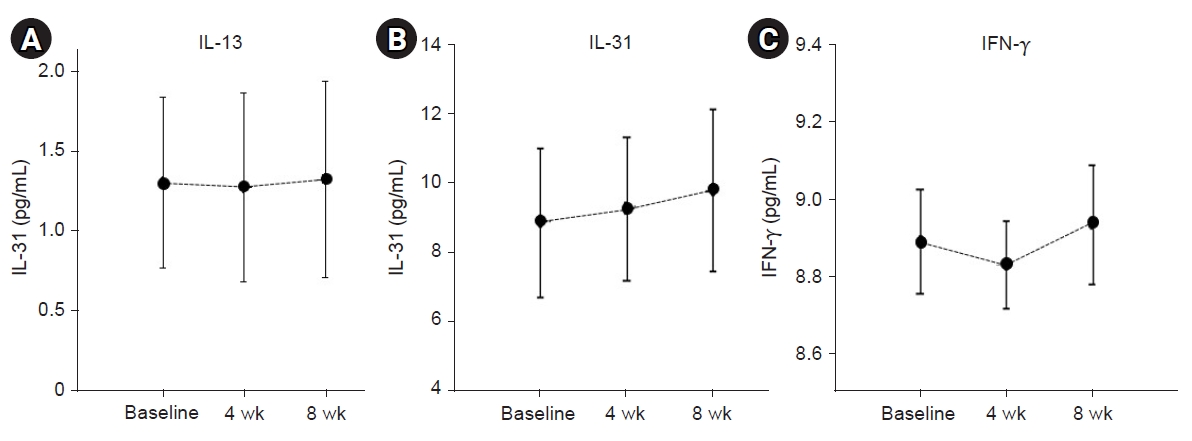
Changes in IL-13 (A), IL-31 (B), and IFN-γ (C) levels at the time of autologous serum intramuscular injection (ASIM) and during follow-up. There were no meaningful changes in the level of IL-13. IL-31 demonstrated a slight increase after ASIM. IFN-γ tended to decrease during treatment and increased during the follow-up period; however, no statistically significant changes were observed between the two time periods. Data are presented as the mean±standard error of the mean of duplicate results from two independent experiments. The statistical significance of differences was assessed by the t-test. IL, interleukin; IFN, interferon.
Discussion
Current standard medical treatment for AD, including topical immunosuppressive therapies, are transient, not completely effective, and can cause side effects. Although the recent use of dupilumab and JAK inhibitors has demonstrated significant effects in management of AD, these drugs have limitations because of their high cost [17]. Additionally, some patients and their family members frequently discontinue treatment because of the associated side effects or drug resistance, leading them to explore unproven treatment methods [18,19]. Therefore, addressing this issue requires the development of novel treatment modalities to improve the clinical course of AD. The present study represents the first clinical trial to assess its effectiveness and safety of ASIM in patients with AD, although ASIM has previously been reported in chronic urticaria [20-23]. Our results demonstrated that ASIM could provide clinical improvements and safe treatment in patients with mild-to-moderate AD who were insufficiently controlled even after previous treatment with topical corticosteroids and oral antihistamines. These findings suggested the potential of ASIM as a new treatment modality for AD.
In 2003, Pittler et al. [6] published a study suggesting that five weekly application of ABT, not ASIM, provides some benefits in the improvement of the six areas and six sign AD severity score in the treatment of AD. However, it did not show favorable results in patient-rated assessments. Our study demonstrated an improvement in the EASI score. Particularly, statistically significant improvements were confirmed not only in the SCORAD score but also in patient-assessed measures, including itching, sleep difficulty, and DLQI score. The SCORAD score encompasses subjective evaluation of itching and sleep difficulty experienced by patients and objective evaluation of skin conditions by physicians. Therefore, our study suggests that ASIM can be more effective in improving quality of life, although it affects the skin lesion itself. Ravaut recommended the use of ABT for dermatological conditions with pruritus and found it to be effective in such conditions [8,24]. Therefore, ASIM can be helpful for patients experiencing pruritus and in those with severe AD, and it can be combined with other drug treatments.
In this study, the clinical symptoms demonstrated the gradual improvement as ASIM treatment period progressed. However, the treatment effect tended to decrease after discontinuing ASIM. This could be attribute to the relatively short duration of the treatment which may have resulted in the inability to sustain the effect over time. In general, autologous serum therapy is administered at weekly intervals for 4 to 8 weeks, whereas in this study, it was only administered for 4 weeks. Additional clinical trials with extended intervention durations are required to gain more insights into the treatment's long-term effects. In present study, the proportions of patients with EASI-50 at weeks 4 and 8 were 40.9% and 18.2%, respectively. In particular, patients who achieved EASI-50 response by week 4 were relatively older and lower peripheral eosinophil counts in the blood compared to the EASI-50 non-responders. These findings suggested that ASIM may be effective in these patients and indicated that ASIM should be considered in the future.
In patients with chronic urticaria, autologous serum therapy has been reported to be more effective in the positive autologous serum skin test group than in the non-positive group [9]. In addition, the shift from Th2 cytokines towards the Th1 immune response was reported when ASIM was administered to urticaria patients who showed a positive response in autologous serum skin test [21,25]. Therefore, ASIM was expected to be effective in AD. In the current study, the effect of ASIM was additionally analyzed based on the results of the autologous serum skin reactions, but no discrepancy in the treatment effect was noted according to the different skin reaction groups. However, statistical interpretation may be limited, due to the small number of positive autologous serum skin tests in patients with AD, accounting for only 18.2% (4 of 22). Further research is required in this regard.
ASIM may cause temporary worsening of symptoms, bruising at the injection site, skin rash and redness, drowsiness, dry mouth, epigastric pain, nausea, and diarrhea [20,22,23,26]. However, when comparing these side effects between the placebo and autologous serum therapy groups, no notable differences in the frequency of side effects were observed between the two groups [6,23]. In the current study, adverse reactions due to ASIM, such as headache, diarrhea, chest discomfort, and myalgia were reported. Although the frequency of these adverse reactions (21.7%) was higher than that reported in other studies, they appeared to be unrelated to the ASIM treatment. Furthermore, no side effects at the injection site were observed in relation to the procedure. As a result, ASIM was considered to be a safe treatment method, however, aseptic management of the specimen must be performed.
The exact mechanism of ABT is unknown, and studies on this topic are rare. Generally, ABT is a type of stimulation or regulation therapy, and it is interpreted that stimulation administered through autologous blood induces counter-regulation and, as a result, induces an immune response opposite to that of the patient's disease, which is helpful for treatment [4]. However, studies have reported that Th2 cytokines transform into Th1 immune responses after ABT, thus suggesting its potential role in modulating allergic immune responses [6,21]. Recently, acupoint autohemotherapy has been reported to be significantly effective in an AD mouse model by regulating Th1/Th2 immune responses [27]. In the current study, IL-13, IL-31, and IFN-γ cytokines were measured before and after ASIM to determine its action mechanism, however no statistically significant changes were observed. Further research is required to clarify this mechanism.
Several limitations were present in this study. First, it underwent analysis as an uncontrolled clinical trial in the absence of a control group. This study was originally planned to include a control group, however, due to difficulties in recruiting participants during the COVID-19 pandemic, control group could not be formed. Thus, statistical analysis had to be performed only in the ASIM intervention group. Second, there was a limitation in accurately evaluating the treatment effect of ASIM because the patient received standard treatment. However, to minimize the influence of the standard treatment and to assess the treatment effect of ASIM, we implemented a 4-week screening and washout period before initiating ASIM therapy. Third, the number of participants enrolled in this study was small and the duration of ASIM intervention was relatively short at 4 weeks. Additional clinical trials with a larger participant pool, extended intervention periods, and randomized placebo-controlled study design are necessary.
In conclusion, this is the first study evaluating the therapeutic efficacy and safety of ASIM in patients with mild-to-moderate AD. Our results suggested that repeated ASIM treatment can improve clinical manifestations in individuals with mild-to-moderate AD, particularly in terms of pruritus and enhancing quality of life. Further clinical trials with large cohorts and additional investigations are needed to elucidate the role of ASIM in AD.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research received support from the “Conditional Approval System of Health Technology” funded by the Ministry of Health and Welfare.
Author contributions
Conceptualization: GSC. Data curation: GSC, JBP, HKK, YHK. Formal analysis: GSC. Funding acquisition: GSC. Investigation: YHK. Methodology: GSC, JBP. Project administration: GSC. Resources: GSC. Supervision: GSC, HKK. Validation: GSC. Visualization: GSC. Writing - original draft: GSC. Writing - review & editing: all authors. Approval of final manuscript: all authors.