Articles
- Page Path
- HOME > Kosin Med J > Volume 39(1); 2024 > Article
-
Case report
Squamous cell carcinoma of the pancreas with a pancreatic intraductal papillary mucinous neoplasm: a case report -
Nam Kyung Lee

-
Kosin Medical Journal 2024;39(1):71-74.
DOI: https://doi.org/10.7180/kmj.23.123
Published online: August 17, 2023
Department of Radiology, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- Corresponding Author: Nam Kyung Lee, MD Department of Radiology, Biomedical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 49241, Korea Tel: +82-51-240-7354 Fax: +82-51-244-7534 E-mail: leenk77@hanmail.net
Copyright © 2023 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 694 Views
- 8 Download
Abstract
- Squamous cell carcinoma (SCC) of the pancreas is very rare. No reports have described SCC accompanied by intraductal papillary mucinous neoplasm (IPMN) of the pancreas. This report presents the first known case of SCC with IPMN of the pancreas in a 71-year-old man, with a focus on radiologic findings. Here, the imaging features of SCC with IPMN of the pancreas were similar to those of IPMN of the pancreas with high-risk stigmata features.
- Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is often related with pancreatic carcinoma, although the malignancy risk was reported to vary considerably with a range between 1.4% and 80.8% [1]. The most invasive carcinomas associated to IPMNs are adenocarcinoma (colloid adenocarcinoma or tubular adenocarcinoma). Because pure squamous cell carcinoma (SCC) of the pancreas is extremely rare, to our knowledge, SCC arising from or concomitant IPMN was also not yet reported. Here, we report the first case of pancreatic SCC with IPMN in the English literature.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2210-021-120). The informed consent was waived because of the retrospective design.
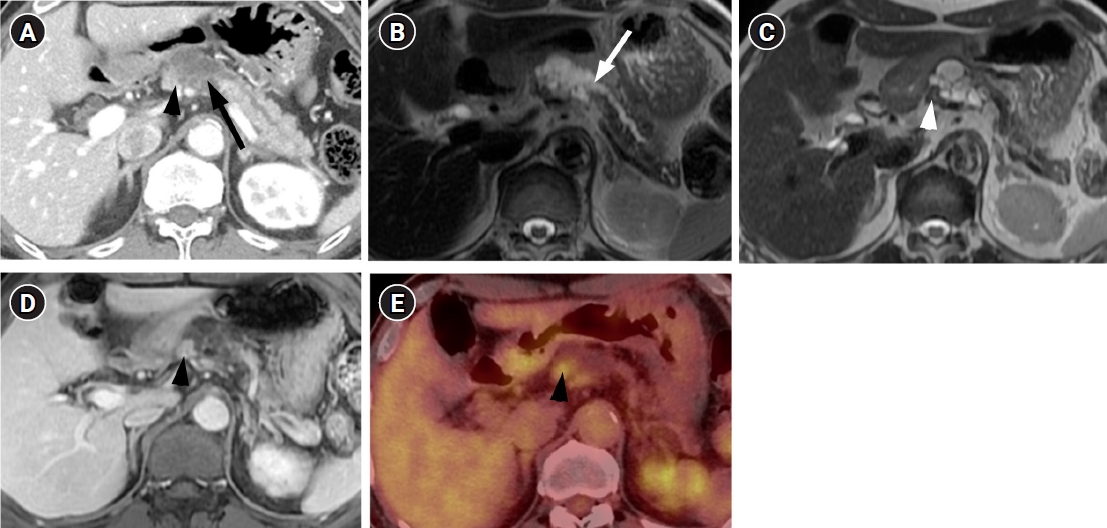
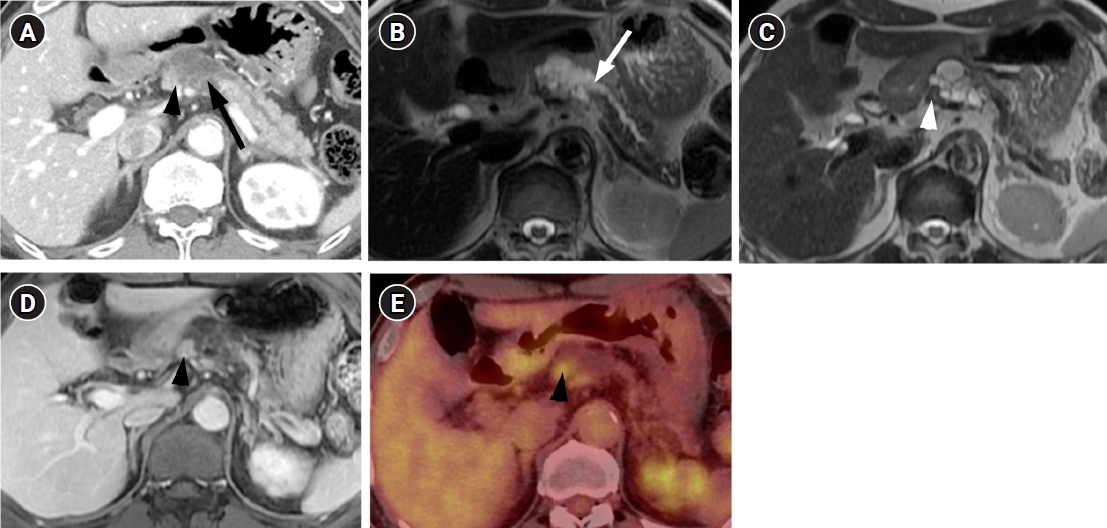
- A 71-year-old man visited our hospital with a complaint of chronic epigastric pain and fatigue. The patient had medical history including diabetes and hypertension. Carcinoembryonic antigen was mildly elevated at 7.7 ng/mL (normal level ≤5 ng/mL). Carbohydrate antigen 19-9 was 21.3 U/mL, which was within the normal range (normal level ≤39 U/mL). Other laboratory findings were within normal range. Computed tomography (CT) showed a 4 cm-sized multiseptated cystic lesion containing mural nodule of ≥5 mm (Fig. 1A). This cystic lesion was suspicious of communication with main pancreatic duct (MPD). Magnetic resonance imaging (MRI) delineated definite connection with MPD. The mural nodule in the cystic lesion showed iso-intensity to pancreas parenchyma on T2-weighted image and enhancement similar to the degree of pancreas parenchyma on contrast-enhanced image (Fig. 1B-1D) Consequently, radiologic diagnosis using CT and MRI was the branch duct type IPMN of the pancreas with high-risk stigmata by using revised 2017 international consensus guideline. 18F positron emission tomography (PET)-CT revealed FDG uptake in the mural nodule of the IPMN (SUVmax of 3.7) (Fig. 1E). Thus, we considered malignant IPMN of the pancreas and performed radical antegrade modular pancreatosplenectomy.
- Histological examination of the resected specimen showed dilated MPD with macrocystic change (Fig. 2A). A defined intramural solid nodule in proximal side of pancreatic duct was also noted (Fig. 2A). Microscopy revealed that the dilated pancreatic duct was lined by gastric type epithelial cells with low- to high-grade dysplasia and forming papillary structures that consist with IPMN of the pancreas (Fig. 2B). The invasive tumor nests of the solid nodule demonstrated keratinized squamous epithelial cells with intercellular bridge and high-grade nuclear atypia that are compatible with SCC features (Fig. 2C). Therefore, the final diagnosis was SCC arising from pancreatic IPMN.
- After surgery, adjuvant chemotherapy was administered once. Subsequently, a gastric ulcer occurred, no additional chemotherapy was performed. The patient has been followed up for 31 months and there was no recurrence during the follow-up period.
Case
- IPMN of the pancreas has malignant potential showing a wide histologic spectrum that ranges from low, intermediate, or high-grade dysplasia and invasive carcinoma [1]. Because management strategy differs based on risk of malignancy, it is critical to accurately predict malignant potentials of IPMN using imaging. Various imaging findings have been evaluated to predict IPMNs with carcinoma, and recently, the 2017 updated guideline for IPMN suggested high-risk stigmata (obstructive jaundice with the cyst located in the pancreatic head, enhancing mural nodule of 5 mm or more, and MPD diameter of 10 mm or more) and “worrisome features” (the cyst size of 3 cm or more, thickened/enhancing walls, and abrupt change of MPD caliber) using CT and MRI [2]. PET-CT was a highly sensitive and accurate modality to distinguish benign from malignant IPMN with mural nodules ≥3 mm. The specificity and positive predictive value were very high (100%), when a SUVmax cutoff of 2.3 was applied [3].
- IPMN with invasive carcinoma means transformation of IPMN to tubular adenocarcinoma, similar to the commonly seen ductal adenocarcinoma that does not arise from IPMN [4]. Adenosquamous carcinoma of the pancreas has rarely been described in association with IPMN of the pancreas [5]. However, to our knowledge, there have been no reported cases of pancreatic SCC arising from or in association with IPMN of the pancreas.
- Primary pancreatic SCC is extremely rare. It accounts from 0.5% to 5% of all exocrine pancreatic tumors. Moreover, primary pancreatic SCC can be diagnosed only after metastasis from other primary organs have been excluded by appropriate examination [6]. Because squamous cells do not exist in the normal pancreas parenchyma, the pathogenesis of pancreatic SCC remains unclear. According to previous literatures, several theories have been proposed: (1) malignant transformation of squamous metaplasia of the ductal epithelium secondary to chronic pancreatitis; (2) transition of preexistent adenocarcinoma to SCC; or (3) the emergency from a precursor progenitor cell of differentiating into squamous or glandular carcinoma [6,7].
- Although clinical manifestation of pancreatic SCC is similar to that of ductal adenocarcinoma, pancreatic SCC have an aggressive behavior with poor prognosis [6,7]. Nevertheless, the imaging findings of pancreatic SCC are also not well known. In according to a previous report, SCC of the pancreas showed hypervascularity compared with ductal adenocarcinomas. Contrast-enhanced CT reported an increased CT attenuation from 35 to 61 Hounsfield units and delayed peripheral enhancement with central necrosis [8]. Whereas, in other case reports written in the Korean language, SCC showed hypovascularity on contrast-enhanced CT and heterogeneous T1 hypointensity and T2 hyperintensity on MRI, which is difficult to differentiate from ductal adenocarcinoma [9]. Lu et al. [10] studied radiologic findings of pancreatic metastasis from lung SCC. The degree of contrast enhancement (hypervascular vs. hypovascular) and the enhancement pattern (peripheral enhancement, homogeneous gradual enhancement) were varied, and in some cases, it was accompanied by dilatation of the biliary-pancreatic duct and vascular invasion. When referring to previous reported imaging features of pancreatic SCC either primary or secondary, imaging findings of pancreatic SCC have more influence depending on the histologic grades or tumor size rather than histological subtype.
- In our case of pancreatic SCC arising from IPMN, an enhancing mural nodule ≥5 mm was seen, suggesting high-risk stigmata in the IPMN, but no specific findings were observed to differentiate from adenocarcinoma arising from IPMN.
- In conclusion, this is the first case report of pancreatic SCC arising from or in association with IPMN of the pancreas. When high-risk stigmata of IPMN of the pancreas are seen in CT or MRI, the possibility of being diagnosed with a rare histologic subtype such as adenosquamous cell carcinoma or SCC arising from IPMN may be recognized. As this is the first reported case of SCC with IPMN, it is expected that further research is needed on pathogenesis, imaging findings, and prognosis.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This work was supported by clinical research grant from Pusan National University Hospital in 2023.
-
Author contributions
All the work was done by NKL.
Article information

- 1. Castellano-Megias VM, Andres CI, Lopez-Alonso G, Colina-Ruizdelgado F. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 2014;6:311–24.ArticlePDF
- 2. Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53.ArticlePubMed
- 3. Takanami K, Hiraide T, Tsuda M, Nakamura Y, Kaneta T, Takase K, et al. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann Nucl Med 2011;25:501–10.ArticlePubMedPDF
- 44. Kim JH, Eun HW, Kim KW, Lee JY, Lee JM, Han JK, et al. Intraductal papillary mucinous neoplasms with associated invasive carcinoma of the pancreas: imaging findings and diagnostic performance of MDCT for prediction of prognostic factors. AJR Am J Roentgenol 2013;201:565–72.ArticlePubMed
- 5. Okamura Y, Sugimoto H, Fujii T, Nomoto S, Takeda S, Nakao A. Adenosquamous carcinoma arising in an intraductal papillary mucinous neoplasm of the pancreas. Pancreas 2010;39:945–7.ArticlePubMed
- 6. Makarova-Rusher OV, Ulahannan S, Greten TF, Duffy A. Pancreatic squamous cell carcinoma: a population-based study of epidemiology, clinicopathologic characteristics and outcomes. Pancreas 2016;45:1432–7.ArticlePubMedPMC
- 7. Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O, Salla C, et al. Squamous cell carcinoma of the pancreas: a systematic review and pooled survival analysis. Eur J Cancer 2017;79:193–204.ArticlePubMed
- 8. Fajardo LL, Yoshino MT, Chernin MM. Computed tomography findings in squamous cell carcinoma of the pancreas. J Comput Tomogr 1988;12:138–9.ArticlePubMed
- 9. Kim JB, Kim MY, Suh CH, Lee KY, Joo YC, Cho JY. Image findings of primary squamous cell carcinoma of the pancreas in patient with chronic pancreatitis: a case report. J Korean Soc Magn Reson Med 2011;15:160–4.Article
- 10. Lu T, Li X, Zhou Y. Pancreatic metastasis from squamous cell lung cancer: computed tomography and magnetic resonance imaging findings. J Int Med Res 2021;49:300060521996188.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite