Transcriptome analysis of the pathogenic ciliate Miamiensis avidus after hydrogen peroxide treatment
Article information
Abstract
Background
The scuticociliate Miamiensis avidus is a highly pathogenic ciliate responsible for serious damage to various organs of aquaculture fish. In particular, the olive flounder aquaculture industry is suffering massive losses due to M. avidus infection. Hydrogen peroxide (H2O2) is one of the most widely used chemicals for scuticociliate treatment. Despite the superior killing effect of H2O2, studies on transcription levels and gene expression changes after H2O2 treatment are limited. We conducted an mRNA transcriptome analysis to compare the differentially expressed gene (DEG) profiles between the ciliate and cyst-like stages of M. avidus after H2O2 treatment.
Methods
We applied DEG profiling to identify DEGs during the ciliate and cyst-like stages of M. avidus.
Results
There were 5,967 DEGs among the 9,075 transcripts identified, and 50 of these DEGs were significantly different (p<0.05). Among these, 21 DEGs were upregulated and 29 were downregulated in the cyst-like stage. The most significantly upregulated genes during the change to the cyst-like stage were cytochrome c oxidase genes. Genes related to the calcium channel were also highly upregulated.
Conclusion
The significant upregulation of cytochrome c gene expression and cytosolic calcium ion channel-related gene expression after H2O2 treatment suggests that ciliate mortality occurred through apoptosis. The formation of the cyst-like stage is considered a temporary form during the process of apoptosis. Information on the gene expression profile of M. avidus in response to H2O2 is expected to contribute to the understanding of the mechanism of action of therapeutic agents against this pathogen.
Introduction
Miamiensis avidus is a highly pathogenic fish parasite with a wide range of aquatic hosts and is one of the most severe threats to many cultured marine fish, especially the olive flounder (Paralichthys olivaceus), as well as marine fish [1]. Recently, devastating outbreaks of M. avidus infection were reported in marine teleost fish along with wild elasmobranchs [2].
It is well known that external stimuli or host immune responses lead to programmed cell death or cyst formation in various species of parasitic protozoa. The harsh conditions like drugs treatment trigger a path of programmed cell death in case of non-cyst-forming protists [3]. Hydrogen peroxide (H2O2) has been shown to play a key role in stress and programmed cell death in previous studies [4].
H2O2 and formalin are the most effective and widely applied chemicals for the treatment of scuticociliates [5] to date. H2O2 has been applied in many non-medical and medical fields owing to its stability in water and strong oxidizing properties. In particular, H2O2 has drawn attention as a suitable biocide in aquaculture, where its decomposition into non-toxic by-products is crucial. Because of these advantages, H2O2 has been approved by the U.S. Food and Drug Administration as a therapeutic agent for several fish pathogens, with applications including in freshwater cultured fish eggs, fingerlings, and against bacterial gill disease in adults [6]. Crosbie and Munday [7] found that Uronema nigricans was susceptible to H2O2 and was lysed completely at 250 ppm (the lowest concentration tested) for 60 minutes. The results in Uronema marinum incubated with H2O2 was similar to that of U. nigricans. When U. marinum was treated with a high concentration of H2O2, it was killed instantly, but it was not completely destroyed at lower concentrations (100 ppm) [8]. These reports indicate that H2O2 can be a potent therapeutic agent against pathogenic ciliates at moderate concentrations.
However, studies on transcriptional level and gene expression changes after H2O2 treatment are still insufficient, despite the excellent killing ability of H2O2. Therefore, we carried out an mRNA transcriptome analysis to compare the expressed gene profiles between the ciliate and cyst-like stages of M. avidus after H2O2 treatment. The comparison of the gene profiles between treated and non-treated groups will provide valuable information for understanding M. avidus destruction by H2O2.
Methods
1. M. avidus culture and cyst-like stage induction
The M. avidus used in this study was obtained from Pukyong National University (Busan, Korea), and was identified as M. avidus using species-specific oligonucleotide primers [9]. For the subculture, M. avidus was inoculated into a culture medium of 2% peptone, 1% yeast extract 0.5% sodium chloride, 10% fetal bovine serum, and 1% penicillin-streptomycin for 3–5 days at 22°C.
Treatment with 0.3% H2O2 in the scutica growth medium (15 mL) with 10% fetal bovine serum induced the cyst-like stage of M. avidus from the ciliated form. Cyst-like structures were confirmed by typical characteristics such as the loss of mobility, formation of an outer membrane, vacuole formation in the cytoplasm, and the loss of cilia. The cyst-like stage of M. avidus was harvested 72 hours after H2O2 treatment.
2. Library preparation and next generation sequencing
The total RNA was extracted using the Maxwell RSC simplyRNA Cells Kit (Promega, Madison, WI, USA) according to the manufacturer's protocols. The RNA concentration and RNA integrity were measured using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) and RNA Nano 6000 Assay Kit on an Agilent Bioanalyzer 2100 System (Agilent Technologies, Santa Clara, CA, USA), respectively. To obtain high-throughput transcript data of M. avidus, cDNA fragments were purified using the TruSeq Stranded Total RNA Sample Prep Kit (Illumina, San Diego, CA, USA) to select fragments of 100–150 bp. Sequencing was performed using an Illumina HiSeq4000 (Opentrons, Opentrons, NY, USA).
3. Sequence analysis and identification
The sequencing data were converted to raw reads by discarding adapters and low-quality reads and obtaining clean reads. Transcriptome assembly was performed based on the merged and clean paired-end reads generated by Illumina Hiseq4000 (Opentrons) using Trinity software. For the identification of functional transcripts, unigenes were searched against the National Center for Biotechnology Information non-redundant database using the BLASTx program with a cutoff E-value less than 1×10-5.
4. Differentially expressed gene analysis
Clean reads were mapped onto the assembled transcripts. The read counts for each gene were obtained from the mapping results and normalized to the number of fragments per kilobase of transcript sequence per million base pairs sequenced (FPKM). The differentially expressed genes (DEGs) were determined based on a difference of at least 2-fold in the FPKM values and a p-value <0.05.
5. Gene ontology enrichment of DEGs
The gene ontology analysis was performed using the sequence annotation tool Blast2GO. After comparing similarities using the default parameters, three main categories (biological processes, cellular components, and molecular functions) were obtained.
Results
1. Sequence analysis and assembly of the transcriptome
Two cDNA libraries (ciliated and cyst-like) were sequenced on an Illumina HiSeq 2500 platform. A total of 98,172,410 and 60,346,680 raw reads were generated from the ciliated (control group) and cyst-like (induced group) databases, respectively (Table 1). After removing low-quality reads, 95,425,100 and 58,045,632 clean reads were obtained for each group, which mapped 97.2% and 96.2%, respectively. The transcripts were assembled into 29,699 single genes with an average length of 374 bp.
2. Gene functional annotation
The presumptive annotation of these transcripts was performed using BLASTx. The putative functions of 9,075 sequences (30.6%) of 29,699 unigenes were identified (Table 2).
3. Most abundantly expressed genes
The gene expression levels were estimated by calculating the abundance of reads in the transcriptome. The 20 most highly expressed transcripts are listed in Table 2. Protein TAR1 was the most abundant gene in the two groups. However, most of the abundantly expressed genes were ribosomal protein genes that are essential for biological metabolism, such as 40S ribosomal protein S14, 60S ribosomal protein L8, Ubiquitin-60S ribosomal protein L40, and 60S acidic ribosomal protein P2. Among these 20 highly expressed genes, only elongation factor 1-alpha and peptidyl-prolyl cis-trans isomerase 1 were co-expressed in both stages (Table 3).
4. Stage-specific gene expression in the ciliated form and cyst-like stage of M. avidus
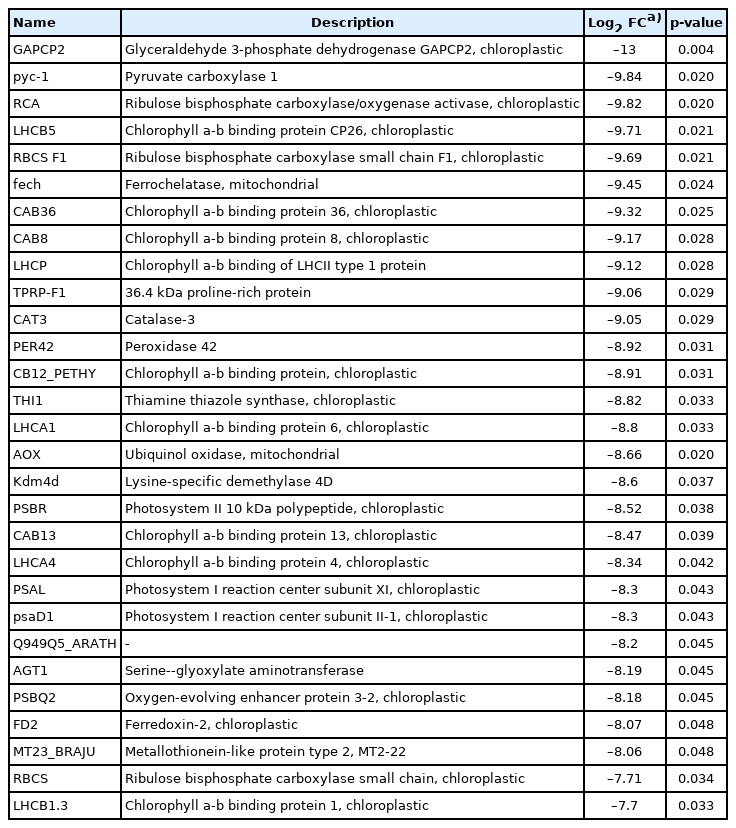
There were 5,967 DEGs among the 9,075 transcripts identified, and 50 of these DEGs were significantly different (p<0.05). Among these, 21 DEGs were upregulated and 29 were downregulated in H2O2 treated group (Tables 4, 5). The most significantly upregulated genes during the change to the cyst-like stage were caricain and cytochrome c oxidase genes. Cytochrome c oxidases are key enzymes in aerobic metabolism and closely related to oxidative stress. Genes related to the calcium channel, calcium transporter, gelsolin, and eIF2 were also highly upregulated. The expression of muscle contraction and cell motility-related genes, such as myosin-6 and myoglobin, were largely upregulated after induction into the cyst-like stage. In addition, the expression of the collagen alpha-1(III) chain and collagen alpha-2(I) chain genes, which encode the major components of collagen that strengthen and support many tissues of the body, also increased. However, the expression of metabolism-related genes, such as glyceraldehyde 3-phosphate dehydrogenase and pyruvate carboxylase 1, decreased after the induction of the cyst-like stage.
5. Gene ontology enrichment analysis of the DEGs
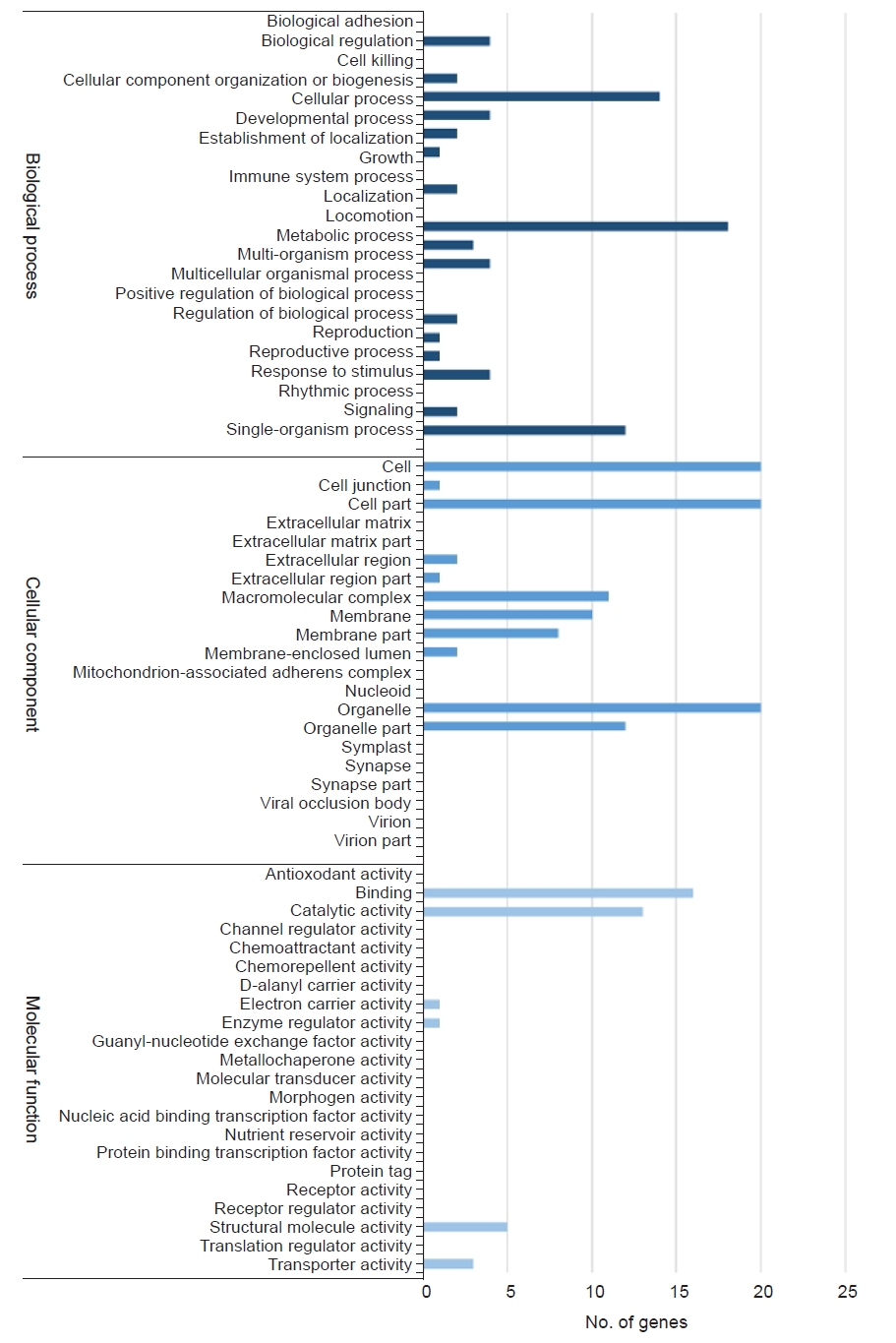
Gene ontology enrichment is generally used to describe the biological roles of genes and their products. All DEGs were mapped to terms in the gene ontology database and compared to the whole transcriptome background to determine the function of the DEGs. The DEGs were classified into three major functional categories: biological processes, cellular components, and molecular functions. In the biological processes category, the DEGs were classified into 22 subcategories and the major DEGs were assigned into “metabolic process,” “cellular process,” and “single-organism process.” Within the cellular component category, the DEGs were classified into 21 subcategories and many DEGs were classified as “cell,” “cell part,” “organelle,” “organelle part,” and “macromolecular complex.” The molecular function categories included 23 subcategories and the subcategories of “binding” and “catalytic activity” were mostly related (Fig. 1).
Discussion
Here, we present comprehensive data on the transcriptome of the M. avidus cilia and the cyst-like stages after H2O2 treatment using the Illumina RNA-seq platform.
One of the survival strategies for single-cell eukaryotes is to transform into a cyst stage in response to unfavorable environmental conditions. This phenomenon is commonly observed in pathogenic and free-living eukaryotes. When we treated M. avidus with H2O2 (0.3%), its shape gradually changed and became a cyst-like round form. This overall structural change was accompanied by several other changes, such as the loss of cilia, vacuole formation, formation of an outer membrane, and the loss of active motion. Because of these changes, we hypothesized that the H2O2 treatment might induce a process similar to cyst formation in M. avidus. The induced cyst-like stage was converted to a small-sized ciliated form when it was returned to the normal media; however, it failed to divide further and eventually died. The life cycle of M. avidus is characterized by three stages: tomite, microstome, and macrostome. Although the tomite stage is non-feeding, it is a fast-swimming stage [10]. Therefore, the cyst-like stage induced by the H2O2 treatment was different from the normal cyst stage. We assumed that the cyst-like stage was an intermediate form during the death process.
Cyst formation is reportedly controlled by intracellular signal transduction pathways that convert environmental signals into gene expression changes [11,12]. Recent studies suggest that the cyclic adenosine monophosphate (cAMP) signaling pathway is probably a common mechanism of cyst formation in protists [13,14]. However, we could not find genes related to the cAMP signaling pathway, and the characteristic responses following encystment, such as autophagy-related gene expression, were not observed. Instead, we identified the caricain gene, which is well known as a class of papain-like cysteine proteases that are central to the immune defense system against invading pathogens or abiotic stress [15,16].
Previous studies have demonstrated that oxidative stress, such as H2O2 treatment, leads to apoptosis-like cell death in single-celled eukaryotes [17,18]. The morphological changes caused by oxidative stress lead to DNA fragmentation, increased vacuolization, nuclear condensation, and cell rounding in single-celled eukaryotes. We also observed some of these changes, such as vacuolization, cell rounding, and immobility.
Oxidative stress caused by elevated reactive oxygen species, such as H2O2, is known to cause programmed cell death through a process similar to apoptosis. Mitochondrial damage by these stimuli leads to excessive cytochrome c release, which can result in DNA damage and apoptosis [19,20]. We also found a significant increase in cytochrome c gene expression, which could explain the high rate of M. avidus death. When Entamoeba histolytica trophozoites were treated with H2O2, apoptosis-like death was induced, and one of the downstream effects was a cytosolic Ca2+ increase [21]. We also found upregulated gene expression related to calcium ion influx, such as the gelsolin and elongation factor 2 genes. Ribosomal protein S60 was highly expressed in both the ciliate and cyst stage. It is known to be involved in the regulation of translational initiation and protein synthesis in response to extracellular stimuli [22]. These findings suggest that the formation of the cyst-like stage of M. avidus after H2O2 treatment followed a similar process of encystment. However, the process was not a natural encystment process, but an intermediate form of cell death. The gene profile expressed during cell death suggests that this process is part of apoptosis.
The most markedly downregulated genes were those involved in glycolysis or gluconeogenesis, namely glyceraldehyde 3-phosphate dehydrogenase and pyruvate carboxylase 1. This was interpreted as a result of metabolic reduction. However, the gene expression levels of catalase and peroxidase were also significantly downregulated. These two genes are well-known enzymes whose optimal substrate is H2O2. It is presumed that the normal response corresponding to apoptosis was not accomplished due to prolonged exposure to H2O2 at a specific concentration. It can also be considered a high mortality effect of H2O2 on M. avidus.
Another point to note from our results was that the expression of chlorophyll-related genes significantly decreased. Most ciliates are heterotrophs, although they can be free-living or symbiotic depending on the food environment. M. avidus is a free-living species in marine environments when it is not parasitic on the fish host. However, chloroplasts have not been reported in ciliate groups, including M. avidus. Therefore, we speculate that the expression of genes with high homology to chloroplast-related genes was the result of the lack of reference genomes that were taxonomically close to M. avidus.
Taken together, the M. avidus cyst-like stage induced by H2O2 showed the upregulation of many apoptosis-related genes. These findings suggest that H2O2 treatment induces apoptosis-like cell death. However, the downregulation of two genes related to H2O2 breakdown suggests further study is required. In addition, a number of chloroplast-related genes were downregulated in the induced cyst-like stage. These results revealed a lack of scutica-related gene information. The comparison of the gene profiles between H2O2-treated and non-treated stages will provide valuable information for understanding M. avidus destruction by H2O2.
Notes
Conflicts of interest
Hee-Jae Cha is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03933725).
This research was a part of the project titled “Omics based on fishery disease control technology development and industrialization (20150242)," funded by the Ministry of Oceans and Fisheries, Korea.
Author contributions
Conceptualization: HK. Data curation: ARL. Formal analysis: HK. Funding acquisition: HJC, MSO. Methodology: HK, ARL, KYJ. Project administration: MSO. Visualization: EJK, MSO. Writing - original draft: ARL, MSO. Writing - review & editing: EJK, HJC, MSO. Approval of final manuscript: all authors.