Agreement of three commercial anti-extractable nuclear antigen tests: EUROASSAY Anti-ENA Profile, Polycheck Autoimmune Test and FIDIS Connective Profile
Article information
Abstract
Background
Detection of antibodies to extractable nuclear antigens (ENAs) is needed for the diagnosis in systemic autoimmune diseases. In this study, we compared three reagents using line immunoblot assay (LIA) or multiplex bead immunoassay for detecting the anti-ENAs.
Methods
A total of 89 sera were tested by 3 different assays: EUROASSAY Anti-ENA Profile (Euroimmune, Germany), Polycheck Autoimmune Test (Biocheck GmbH, Germany), and FIDIS™ Connective Profile (Biomedical Diagnostics, France). The following individual ENAs were investigated: Sm, SS-A (Ro), SS-B (La), Scl-70, Jo-1 and RNP. We reviewed medical records to investigate the discrepant results among three methods.
Results
Overall percent agreements were 96.1% between EUROASSAY Anti-ENA Profile and FIDIS™ Connective profile; 90.4% between EUROASSAY Anti-ENA Profile and Polycheck Autoimmune Test using the manufacturers’ cutoff; 96.4% between EUROASSAY Anti-ENA Profile and Polycheck Autoimmune Test using a upward cutoff; 90.4% between FIDIS™ Connective profile and Polycheck Autoimmune Test the manufacturers’ cutoff; and 96.4% between FIDIS™ Connective profile and Polycheck Autoimmune Test a upward cutoff.
Conclusions
The three assays showed excellent agreement with each other. With appropriate cutoff, the all three assays for six of the anti-ENA tests investigated in this study can be used in clinical laboratories for detecting the anti-ENAs.

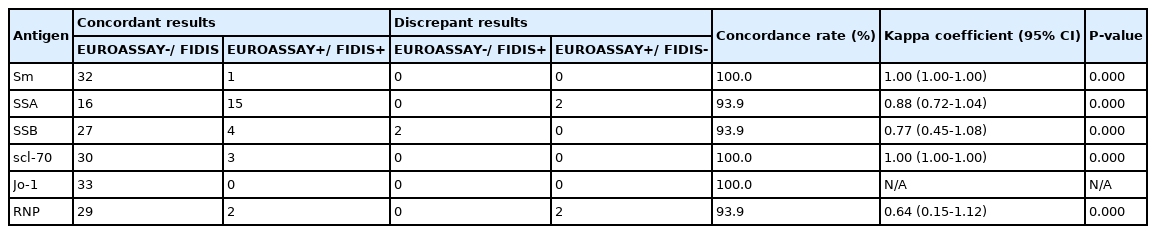
Comparison of the results for antibodies to extractable nuclear antigens by EUROASSAY Anti-ENA Profile and Polycheck Autoimmune Test (N = 89)
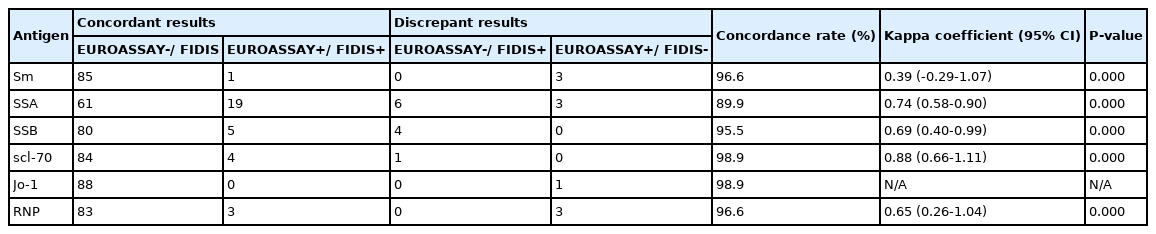
Comparison of the results for antibodies to extractable nuclear antigens by EUROASSAY Anti-ENA Profile and FIDIS Connective Profile (N = 89)
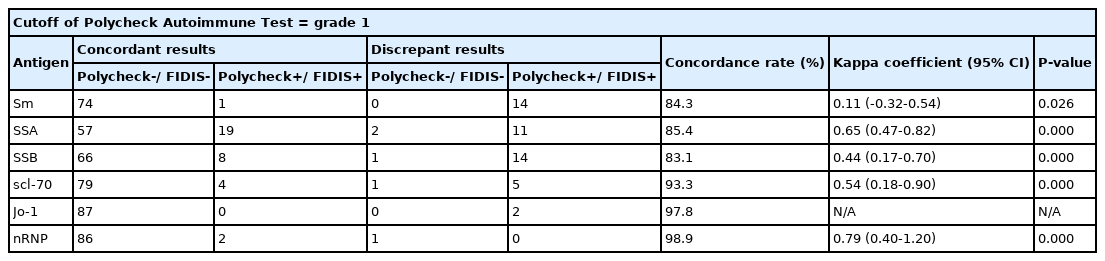
Comparison of the results for antibodies to extractable nuclear antigens by Polycheck Autoimmune Test FIDIS Connective Profile (N = 89)