Heterotopic ovarian hilus cells of the salpinx: a case report and literature review
Article information
Abstract
Ovarian hilus cells (OHCs), a counterpart of testicular Leydig cells, are usually found in the ovarian poles and produce androstenedione. Their origin remains a matter of debate, although OHCs are assumed to come from the adrenogenital primordium. OHCs are rarely observed around the poles of the ovary, including the mesoovarium, stroma of the salpinx (perisalpinx), and the wall of paratubal cysts. Their clinical and pathological characteristics are not well-known because of their rarity. Herein, we present a case of ectopic OHCs in a 48-year-old woman. The patient underwent total hysterectomy and bilateral salpingectomy for vaginal bleeding due to multiple leiomyomas. We incidentally found OHCs in the stroma of the infundibulum of the salpinx, just beneath the tubal epithelium. Their size was less than 1 mm, and they were composed of large cells with central round nuclei and abundant clear or granular cytoplasm. OHCs share morphological and immunohistochemical profiles with ectopic adrenal glands, and the differential diagnosis is sometimes difficult. They do not exhibit microscopic encapsulation or the normal adrenal cortex zonation pattern. The patient was discharged and did not show any abnormal findings during 19 months of follow-up. Analyzing the characteristics of testicular Leydig cells will help understand how OHCs develop and why heterotopic OHCs occur in and around the salpinges.
Introduction
Ovarian hilus cells (OHCs), also called interstitial (Leydig) cells or ovarian Leydig cells (OLCs), are commonly present in the stroma of the female gonad during second trimester and most of them degenerate until term [1,2]. Their origin is controversial and are proposed to come from undifferentiated ovarian mesenchyme, unmyelinated nerves, and perivascular or perineural fibroblasts [3,4]. OHCs, counterpart of testicular Leydig cells, may be present as the epoophoron, paroophoron, and Gartner’s duct along the regressed mesonephric duct (Wolffian duct) [5,6]. Heterotopic OHCs are rare (0.52%, 12/2,299), while eutopic OHCs are common (80%) [2,7]. Herein, we present a case of heterotopic hilus cells that were detected in hysterectomy and bilateral salpingectomy specimens from a 48-year-old woman who had multiple leiomyomas. We compared this case with other reported cases of heterotopic hilus cells found in the salpinx or parasalpingeal areas and reviewed them to elucidate the characteristics of heterotopic hilus cells.
Case
Ethical statements: The Institutional Review Board of Haeundae Paik Hospital approved the study (IRB No. HPIRB 2022-07-001), and informed consent was waived due to the retrospective nature of study and the type of publication.
A 48-year-old Asian woman presented with vaginal spotting and menorrhagia. According to the GTPAL (gravida, term, preterm, abortion, and living) system, she was 2-0-1-2 and had her last menstruation 2 weeks prior. The patient had been treated for rheumatoid arthritis. She denied any previous history of surgery. Transvaginal ultrasonography revealed a 6.5×5 cm-sized solid mass in the uterus. Therefore, clinical diagnosis of leiomyoma was made. The level of cancer antigen 125 was 23.4 U/mL (normal range: –35 U/mL). The patient underwent Da Vinci Robot-assisted laparoscopic vaginal hysterectomy with bilateral salpingectomy. On the operation field, two solid masses were found. The first one, measuring 8cm, was located at the uterosacral ligament. The second intrauterine mass measured 4 cm. Both adnexae were grossly nonspecific.
Microscopic findings of the solid masses were consistent with leiomyoma. A low-grade squamous intraepithelial lesion was also found in the uterine cervix. The outer aspect of bilateral salpinges was smooth (Fig. 1). The lumens of the salpinges were patent with thinned salpingeal walls. Wall thickening of the right infundibulum salpinx was focally observed at a low magnification (Fig. 2A). When observing the thickened wall at a high magnification, nests of epithelial cells were incidentally found. The epithelial cells were intermixed with myofibers of the myosalpinx (Fig. 2B). Unmyelinated nerve fibers and vessels were noted near them, but they were not located in the perineural or intraneural area. These cells formed a non-encapsulated nodule measuring 835×641 μm just beneath the tubal epithelium. Some clusters of regularly sized round or oval cells had uniformly round vesicular nuclei with inconspicuous nucleoli and acidophilic granular and sometimes vacuolated cytoplasm (Fig. 2C). Capsulation, lipofuscin pigment or Reinke crystal was absent. Immunohistochemistry for inhibin-alpha, and Melan-A was positive (Fig. 2D), while that of calretinin was negative, and CD56 was expressed in the periphery of the cellular nest (Fig. 2E). Mesonephric duct remnant was found near hilus cells (Fig. 2F). Entire sections of both fallopian tubes and ovaries were embedded according to the SEE-FIM protocol, but no other hilus cells were found.

Gross findings. Anterior (A) and posterior side (B) of the fresh right fallopian tube following hysterectomy with bilateral salpingectomy showing several fimbrial cysts at the end of the right fallopian tube. The external surface of the bilateral salpinges was smooth. A couple of subserosal small leiomyomas were also noted.
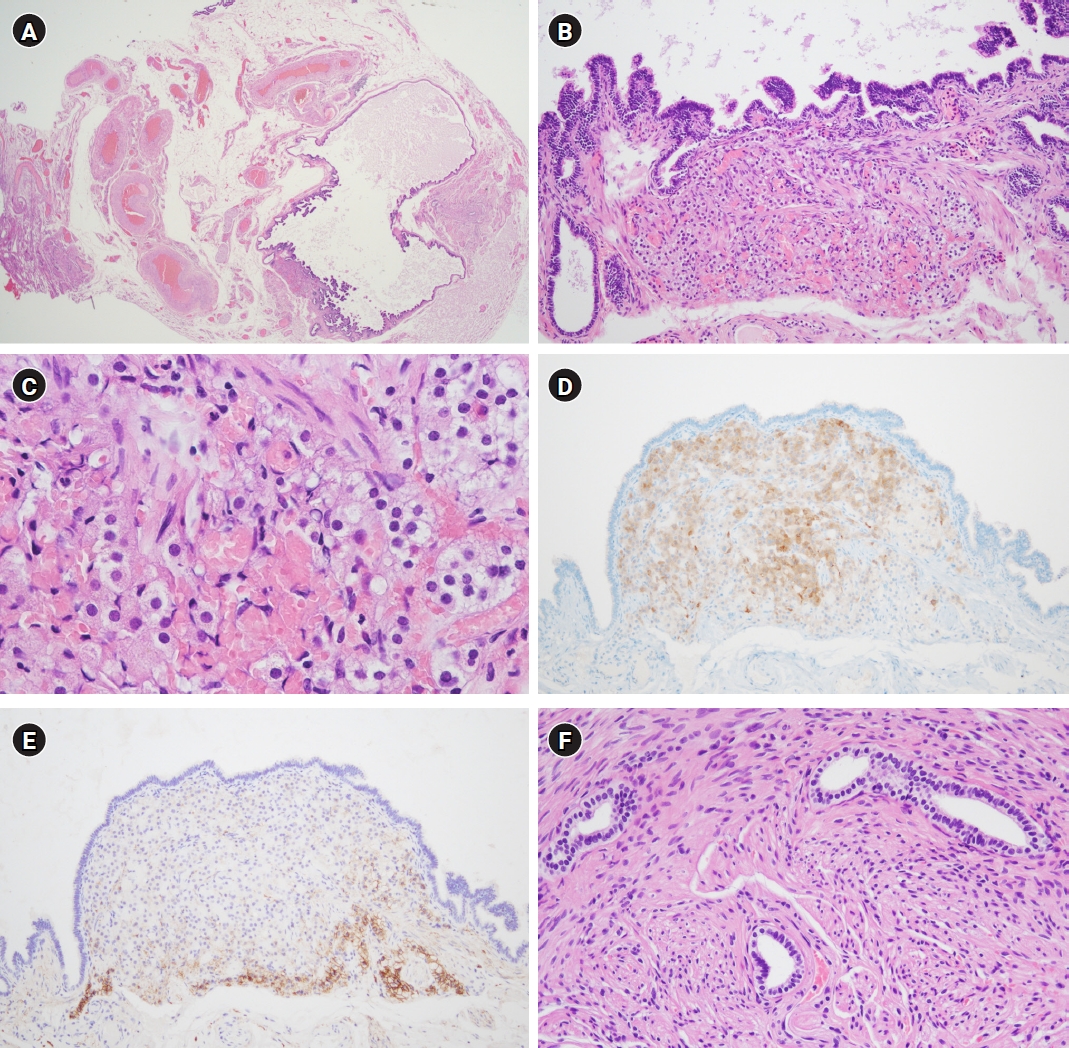
Microscopic and immunohistochemical findings. (A) Under low-power magnification, the infundibulum of the fallopian tube had a thinned myosalpinx and tertiary branch of plicae. Thick-walled vessels and bundles of nonmyelinated nerves were noted in the mesosalpinx (hematoxylin and eosin stain [H&E], ×12.5). (B) Some nests of epithelial cells are present just beneath the tubal epithelium and admixed with smooth muscle fascicles of the myosalpinx. These oval cells had abundant eosinophilic cytoplasm and were arranged in a trabecular pattern with sinusoidal blood vessels. They were just next to the blood vessels (H&E, ×100). (C) Oval or round cells had round nuclei, some of which had inconspicuous nucleoli. Cells on the left side showed vacuolated cytoplasm (H&E, ×400). (D) The cells were immunoreactive to antibodies produced against inhibin-alpha (inhibin-alpha immunohistochemistry, ×100). (E) CD56 was expressed in the periphery of the nest of hilus cells (CD56 immunohistochemistry, ×100). (F) Wolffian duct remnants, showing ducts lined by cuboidal cells and wrapped with smooth muscle, were identified in the mesosalpinx around ectopic hilus cells (H&E, ×200).
Based on this, a final diagnosis of low-grade squamous intraepithelial lesion of the uterine cervix and multiple leiomyomas of the uterosacral ligament and myometrium was made. In addition, the epithelial cellular nest of the right salpinx was considered as heterotopic OHCs considering the histologic and immunohistochemical findings. The patient was discharged on the 5th postoperative day. Follow-up 19 months later indicated that she was well and was receiving treatment for rheumatoid arthritis.
Discussion
OHCs are a counterpart of testicular Leydig cells, and thus are referred to as OLCs [8]. Therefore, analyzing Leydig cells and development of the urogenital tract may be helpful to understand OHCs. Leydig cells rarely proliferate but they are not “static.” There is a hypothesis that fetal Leydig cells degenerate in the neonatal and prepubertal periods but persist and account for 20% of Leydig cells, and adult Leydig cells become predominant in the interstitial space after puberty [9]. Hilus cells are present at birth, disappear during childhood, reappear at puberty and persist after menopause [3]. It is possible that there are also two types of OHCs and the periodic developmental changes of fetal and adult Leydig cells may explain why OHCs temporarily disappear during the childhood in human’s life. The origin of OHCs is controversial, but are known to originate from the adrenogenital primordium, coelomic epithelium, mesonephros, neural crest or multiple and distinctive progenitor cells from early microvessels [10]. According to Defalco et al. [10], there are two progenitor lineages of fetal Leydig cells; one that is positive for musculoaponeurotic fibrosarcoma oncogene family protein B expression and originates from the coelomic epithelium and specialized cells along the gonad-mesonephros border lineage. Another that is positive for vascular cell adhesion molecule 1 and originates from perivascular cells. Interestingly, ectopic Leydig cells are associated with recruited vessels, which supports that Leydig cell differentiation is related to vasculature as well as fibroblast growth factor 9 [10]. This may support the close anatomic correlation between heterotopic hilus cells and vascular or nervous system. Carrasco-Juan et al. [6] reported that OHCs are normally located along the ovary (hilum, medulla, or cortical stroma) and in the mesoovarium, fimbrial stroma of the salpinx, mesosalpinx, or endoneurium and subperineurium of the nerves therein. Our case is “heterotopic” according to their definition. However, the mesothelium and Müllerian and Wolffian ducts are closely related during fetal development, and thus the classification into heterotopia or eutopia is debatable.
We reviewed 33 cases of heterotopic hilus cells of the salpinx or mesosalpinx, which had been reported (Supplement Table 1) [4,6,7,11-19]. The recent use of the SEE-FIM protocol seems to have increased the discovery of heterotopic hilus cells of the salpinx and mesosalpinx since 2010. The symptoms of the patients were related to other diseases including leiomyoma, endometrial carcinoma or mature teratoma, and not hormones. The age of the patients ranged from 42 to 72 because young adults rarely undergo salpingectomy or tubal ligation, and the number of young adult specimens may be extremely low. Except for the cases with no information (18 cases), heterotopic hilus cells were found on both sides (7 cases), the right salpinx (4 cases), or the left salpinx (4 cases). The fimbria(e) or fimbrial cyst was the most common site (16 cases), possibly due to the close proximity of the ovary and salpingeal fimbria(e). In addition, in each one case, hilus cells were found in the midportion of the salpinx, paratubal cyst, or paraisthmus. The mesosalpinx was also one of the sites where heterotopic hilus cells were found (6 cases). Reinke crystal and lipofuscin pigment, which are known as important pathologic features of hilus cells, rarely appeared in three and three cases, respectively. Although they are characteristic, they are not always observed, so their absence cannot exclude OHCs. It is interesting that anatomic relation with nerves or vessels was found in fourteen cases.
The main pathological differential diagnosis is ectopic adrenal rest. The cytological findings of hilus cells are much similar to those of the zona reticularis of the adrenal cortex. Immunohistochemical findings, including those of inhibin-alpha, Melan-A, and calretinin, were identical to those of the adrenal cortex. Interestingly, both of them produce steroid hormones. The report of Hatano et al. [20] that adrenal glands and gonads develop from a common primordium during embryogenesis of rats may explain the similarities between them, and this may explain why they share morphology and function. The differential diagnosis was based on observations under lower-power view, including capsulation and zonation, which are observed in the ectopic adrenal gland but not in hilus cells [2]. Another differential diagnosis is perineural or intraneural invasion of metastatic carcinoma. Camacho-Partida and Ortiz-Hidalgo [18] reported a case of OLCs mimicking perineural invasion of endometrial adenocarcinoma. Immunohistochemical profiles of calretinin positivity, inhibin-alpha positivity and cytokeratin negativity point to a diagnosis of OLCs.
Because the number of reported case of OHCs is small, we did not find any report of OHC cases with clinical significance. It is difficult to determine the amount of androstenedione produced by OHCs and the symptom they induce. Like other heterotopic tissues, hyperplasia or neoplasms can occur in OHCs. In addition, OHCs may be related to infertility if they are located in the salpingeal stroma and make the salpingeal lumen narrow.
In conclusion, we showed that heterotopic OHCs are rare entity and are found almost incidentally. The clinical significance of OLCs is usually not critical and is generally not related to endocrine symptoms. They can help in the pathological differential diagnosis of conditions, such as heterotopic adrenal rests and perineural invasion of metastatic carcinoma. Careful assessment of histology, including anatomical association with nerves or vessels, and immunohistochemistry can help achieve a correct diagnosis. In addition, more research about OLC as well as testicular Leydig cells is needed to clarify their characteristics.
Supplementary material
Supplementary materials are available at https://doi.org/10.7180/kmj.23.120
Supplementary Table 1. Summary of clinical and pathological characteristics of heterotopic hilus cells in the salpinx and mesosalpingeal areas
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
All the work was done by BK.
