Risk factors for central and lateral lymph node metastasis in papillary thyroid carcinoma
Article information
Abstract
Background
Lymph node metastasis (LNM) is commonly observed in papillary thyroid carcinoma. This study aimed to investigate the risk factors for LNM in patients with papillary thyroid carcinoma.
Methods
The clinicopathological factors of 417 patients were investigated, and differences according to the presence or absence of LNM were evaluated.
Results
LNM was associated with age <55 years, male sex, tumor size >10 mm, multiple and bilateral tumors, tumor involving the lower pole or entire lobe, lymphovascular invasion (LVI), perineural invasion (PNI), and extrathyroidal extension (ETE). Univariable and multivariable analyses showed that age <55 years, male sex, tumor size >10 mm, LVI, and ETE were related to central LNM. Male sex, tumor size >10 mm, and LVI were correlated with lateral LNM (p<0.05). Compared to central LNM, more lymph nodes were involved in metastases and the metastatic tumors were larger in lateral LNM. Extranodal extension (ENE) was more commonly observed in lateral LNM (p<0.001) and was associated with tumor size >10 mm, multifocality, PNI, ETE, and the absence of lymphocytic thyroiditis (p<0.05).
Conclusions
Younger age, male sex, tumor size >10 mm, LVI, and ETE were risk factors for central LNM, while male sex, tumor size >10 mm, and LVI were risk factors for lateral LNM. ENE was more commonly observed in lateral LNM, and tumor size >10 mm, multifocal tumors, PNI, ETE, and tumors unrelated to lymphocytic thyroiditis were risk factors for ENE.
Introduction
Thyroid cancer is a common malignancy of the endocrine system, and the diagnosis and treatment rates have increased in Korea due to the activation of health examinations. Among thyroid cancer, 80% to 90% are papillary thyroid carcinoma (PTC) [1]. Although most patients with PTC have a good prognosis with a long-term survival rate of over 90% [2], PTC affects the patient's quality of life with respect to local metastasis or recurrence. Regional lymph node metastasis (LNM) is the major factor in recurrence and reoperation [3,4]. LNM is common in PTC, with an incidence of 30% to 80% [4].
In general, for PTC with central neck LNM, thyroidectomy and central compartment lymph node dissection are recommended. However, it is not easy to predict LNM, and preoperative ultrasound can detect only 20% to 31% of central neck LNMs [5]. According to the American Thyroid Association guidelines, prophylactic central compartment lymphadenectomy is not recommended due to the low reliability of preoperative examinations [6].
In the course of routine pathology, we may find cases with significant LNM despite the small size of the tumor, and conversely, cases where no LNM is observed despite large tumor size. LNM usually develops first in the central compartment and later progresses to the lateral compartment [5]. However, skip metastasis, which skips the central compartment and immediately metastasizes to the lateral compartment, is also often observed. Therefore, analyzing and accurately predicting the risk factors for LNM can help to plan appropriate surgery and management and help to prevent a recurrence.
The aims of this study were to elucidate the clinicopathological differences of PTC according to the presence or absence of LNM and investigate factors that can predict LNM.
Methods
Ethical statements: Ethical approval: This study was approved by the Institutional Review Board of Presbyterian Medical Center (IRB No. 2022-01-006). Patient consent was waived because this study was a retrospective review of pathological diagnoses in an operated sample, which had no effect on patients.
1. Patient selection
Patients who underwent surgery and histopathological examination for PTC at the Presbyterian Medical Center from March 2019 to December 2021 were selected through medical records. All age groups and tumor sizes were included. Classic type cases, as well as other types, were included. Patients with coexisting carcinomas such as follicular carcinoma were excluded. Cases with missing data were not included. The surgical policy was based on lobectomy or total thyroidectomy along with central lymph node dissection. Central lymph node dissection was performed prophylactically, regardless of preoperative ultrasound findings. Lateral lymph node dissection was performed for therapeutic purposes only if preoperative lateral LMN was suspected.
2. Clinical parameters
Data on age and sex were collected. Age was classified into two groups based on an age of 55 years according to the criteria for staging differentiated thyroid cancer in the American Joint Committee on Cancer Cancer Staging Manual 8th edition [7].
3. Histopathologic parameters
Surgically removed specimens were examined by four pathologists and the following histopathologic parameters were evaluated: tumor size, location, multifocality, bilaterality, histologic subtype, lymphovascular invasion (LVI), perineural invasion (PNI), extrathyroidal extension (ETE), LNM, extranodal extension (ENE), and lymphocytic thyroiditis. The size of the tumor was evaluated by dividing them into two groups: those smaller than or equal to 10 mm and those larger than 10 mm. In the case of multiple tumors, only the size of the largest tumor was collected. Multifocality was defined as two or more tumors. LNM was subgrouped into central and lateral LNMs. The diagnosis of lymphocytic thyroiditis was made when diffuse lymphocyte infiltration and fibrosis were observed throughout the thyroid gland regardless of the presence or absence of thyroid parenchymal atrophy. Immunohistochemical studies were performed to evaluate LVI and PNI.
4. Detection of LVI, PNI, and BRAF V600E mutation using immunohistochemistry
Immunohistochemistry was performed on 4-µm-thick sections of formalin-fixed, paraffin-embedded tissue blocks. The antibodies used were mouse monoclonal antibody to CD34 (NCL-L-END, Novocastra; Leica Biosystems, Wetzlar, Germany), mouse monoclonal antibody to podoplanin (D2-40, Cell Marque; Sigma-Aldrich, St. Louis, MO, USA), rabbit polyclonal antibody to S-100 (NCL-L-S100p, Novocastra), and mouse monoclonal antibody to anti-BRAF V600E (VE1; Ventana Medical Systems, Oro Valley, AZ, USA). The Bond-Max automated immunostainer was used for CD34 and S-100 staining. The automated Ventana BenchMark immunostainer (Roche, Basel, Switzerland) was used for podoplanin and anti-BRAF V600E staining. Staining was performed according to each protocol. Immunoreactivity was visualized using the Bond Polymer Refine Detection Kit (Leica Biosystems) and the OptiView IHC DAB Kit (Ventana Medical Systems). Tissues were counterstained with hematoxylin II and bluing reagent for 4 minutes. Normal thyroid tissue was used as the negative control for anti-BRAF V600E staining.
5. Statistical analysis
All statistical analyses were performed using SPSS version 28.0 software (IBM Corp., Armonk, NY, USA). Data were compared using the chi-square test, Student t-test, and binary logistic regression. Regression analysis was performed to evaluate the risk factors for central LNM and lateral LNM, and the central and lateral LNM groups were compared with the non-metastatic group. Multivariable analysis was performed on meaningful data in the univariable analysis. In all statistical methods implemented, statistical significance was defined as p-values of less than 0.05.
Results
1. Clinicopathologic findings
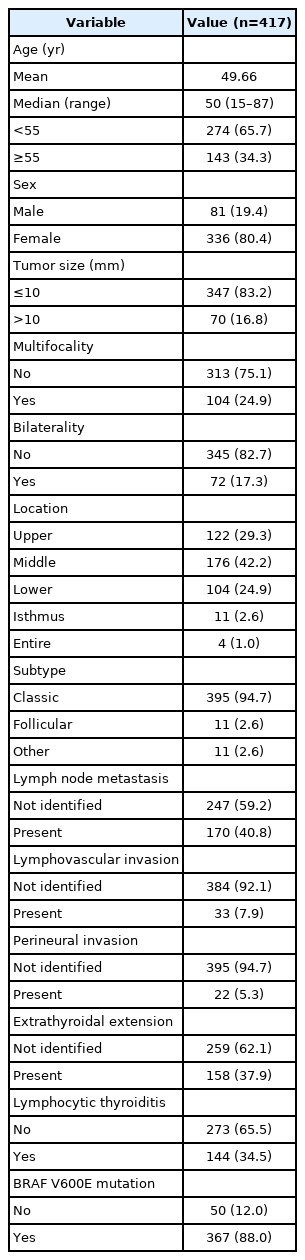
As shown in Table 1, a total of 417 patients with PTC were enrolled. The mean age and median age were 49.66 and 50 years, respectively (range, 15–87 years). Of the total patients, 274 (65.7%) were under the age of 55, and 143 (34.3%) were 55 years of age or older. There were 81 males (19.4%) and 336 females (80.4%). There were 374 and 70 cases with a tumor size smaller than or equal to 10 mm and larger than 10 mm, respectively. One hundred and four cases showed multifocality and 72 cases showed bilaterality. The most common tumor location was in the middle portion (176 cases, 42.2%), followed by the upper pole (122 cases, 29.3%) and the lower pole (104 cases, 24.9%). The classic subtype was the most common with 395 cases (94.7%), and there were 11 cases (2.6%) each of the follicular subtype and other subtypes. The other subtypes included seven cases of tall cell variant, two cases of solid variant, and two cases of oncocytic variant. LNM was observed in 170 cases (40.8%), and LVI and PNI were observed in 33 cases (7.9%) and 22 cases (5.3%), respectively. ETEs were observed in 158 cases (37.9%), and all but one were microscopic extensions. In one case, tracheal invasion was observed at the time of surgery and pathologic confirmation was made. There were l44 (34.5%) cases with lymphocytic thyroiditis. In the immunohistochemical analysis for BRAF V600E mutation, 367 cases (88.0%) showed positive findings.
2. Comparison of PTC with or without LNM
There were 170 (40.8%) cases with LNM and 247 (59.2%) cases without LNM. LNM was observed more frequently and was statistically significant in the following cases: patients under the age of 55 years, male sex, tumor size >10 mm, multifocal tumors, bilateral tumors, tumors involving the lower pole or entire lobe, LVI, PNI, and ETEs (Table 2). There was no significant correlation between tumor subtype, lymphocytic thyroiditis, and BRAF V600E mutation with LNM.
3. Central and lateral LNM features
Of the total 170 cases with LNM, 22 (12.9%) spread to the lateral compartment of the neck, while 148 (87.1%) were localized to the central compartment. There was no case of “skip metastasis.” Table 3 shows the differences between the central and lateral LNM groups. The average number of lymph nodes involved with metastatic tumors was significantly different between the lateral LNM group and the central LNM group (9.96±6.56 vs. 2.81±2.27, p<0.001). The mean greatest metastatic tumor size was larger in the lateral LNM group (14.18±6.79 mm vs. 3.24±2.80 mm), showing statistical significance (p<0.001). An ENE was observed in 17 cases (77.3%) and 45 cases (30.4%) in the lateral and central LNM groups, respectively, and there was a statistically significant difference (p<0.001).
4. Risk factors for central LNM
As shown in Table 4, central LNM was correlated with the following factors in the univariable analysis: Age <55 years, male sex, tumor size >10 mm, bilaterality, LVI, and ETEs. However, in the multivariable analysis, age <55 years, male sex, tumor size >10 mm, LVI, and ETEs were associated with central LNM.
5. Risk factors for lateral LNM
Table 5 shows the results of the analysis of the risk factors for lateral LNM. Lateral LNM was correlated with male sex, tumor size >10 mm, multifocality, bilaterality, LVI, PNI, ETEs, and BRAF V600E mutation in the univariable analysis. The multivariable analysis showed that male sex, tumor size >10 mm, and LVI were associated with lateral LMN.
6. Risk factors of for ENE
The analysis of risk factors for ENE is shown in Table 6. In the univariable analysis, ENEs showed significant associations with tumor size >10 mm, multifocality, bilaterality, PNI, ETEs, and the absence of lymphocytic thyroiditis. The multivariable analysis showed that tumor size >10 mm, multifocality, PNI, ETEs, and the absence of lymphocytic thyroiditis were associated with ENEs.
Discussion
The purpose of this study was to investigate the factors that could predict LNM. Although PTC is known to be an indolent tumor, LNM can occur at an early stage [5]. LNM increases the risk of local recurrence and reoperation and reduces the quality of life [4]. It is common that central LNM precedes lateral LNM, but skip metastasis is also found. Therefore, recognizing the risk factors of LNM and providing appropriate treatment in the initial operation is important for patient prognosis.
Previous studies showed that factors such as sex, age, ETE, and larger tumor size, especially tumors larger than 10 mm, were associated with LNM [3-5,8]. Kim et al. [9] also showed that the number of tumors was associated with LNM. In this study, LNM was detected in 40.8% of the patients, which was consistent with reports in the literature. LNM was observed at younger ages, in males, in tumor sizes >10 mm, in multiple and bilateral tumors, and in cases with LIV, PNI, and ETEs. In the predictive model, age <55 years, male sex, tumor size larger than 10 mm, LVI, and ETEs were associated with central LNM, and male sex, tumor size >10 mm, and LVI were associated with lateral LNM.
The relationship between tumor location and LNM remains controversial. For tumors located in the upper pole, it has been reported that metastases to the ipsilateral lateral cervical lymph nodes were more common because of the physically close location [4,10]. However, a previous study found no association between tumor location and LNM [11]. This study showed more frequent LNM in tumors located in the lower pole. However, in the predictive model, tumor location and central and lateral LNM were not correlated.
The follicular type of PTC is known for its less aggressive behavior and better prognosis. The tall cell and solid subtypes are known to be more aggressive and have frequent lymph node metastases. No differences in LNM between subtypes were identified in this study. Most of the cases in this study were the classic type and there were a few cases of other types, so there was a limit to the comparisons that could be made. Whether BRAF V600E mutations are related to LNM is still controversial [12-15]. In this study, LNM was not associated with BRAF V600E mutations.
This study also revealed that lateral LNM exhibited a more aggressive pattern compared to central LNM, showing a higher number of involved lymph nodes, a larger size of the metastatic tumors, and more frequent ENEs.
ENEs were frequently observed in lateral LNM and were associated with tumor sizes >10 mm, multifocality, PNI, ETEs, and the absence of lymphocytic thyroiditis. Multifocality is thought to be associated with more aggressive behavior in PTC [16]. ENEs also seem to be associated with a poor prognosis [17,18]. Zhou et al. [17] reported that patients with ENEs had lower recurrence-free survival rates than patients without ENEs and suggested that ENE was an independent prognostic factor in PTC. Genpeng et al. [18] also showed that ENEs were associated with poor prognosis and proposed a novel staging system that integrated extranodal expansion into the classification of tumors, lymph nodes, and metastases. This study was a retrospective study of the patients who underwent surgery within a short period of 2 years and 10 months, so there was a limit to revealing the relationship between ENEs and the factors predicting prognosis, such as recurrence-free survival. Further investigation on the prognostic relevance of an ENE is needed.
PNI is thought to be invasive in several carcinomas. Rowe et al. [19] showed that nerve density was increased in PTC, and PNI was positively associated with ETEs. Although microscopic ETEs did not have a significant effect on the prognosis of patients with PTC [20], studies have shown that ENEs were significantly higher in tumors with ETEs [21,22]. ENEs were observed to be highly associated with PNI and ETEs in this study and these findings are consistent with those of previous studies.
The relationship between lymphocytic thyroiditis and PTC is complex. There is evidence that lymphocytic thyroiditis increases the incidence of PTC [1], but lymphocytic thyroiditis is also associated with a better prognosis for patients with PTC [23,24]. In this study, ENEs were more frequently observed in the group of patients without lymphocytic thyroiditis. This result supports the results of previous studies on the prognostic relationship between LT and PTC, given the results of this study that ENEs were associated with aggressive factors including PNI and ETEs in PTC.
This study has some limitations. First, this study is a retrospective study of the experience of a single institution. Therefore, a sufficient number for each subtype was not included. In addition, due to the lack of sufficient investigation for preoperative examination, the difference between the group with and without preoperative central LNM diagnosis was not compared. Further research will be needed for evaluation of the significance of prophylactic central lymph node dissection.
In summary, the factors predicting central LNM were age <55 years, male sex, tumor size >10 mm, LVI, and ETEs. Male sex, tumor size >10 mm, and LVI were considered predictive factors for lateral LNM. ENEs were more frequently observed in lateral LNM, and tumor sizes >10 mm, multifocality, PNI, ETEs, and tumors unrelated to lymphocytic thyroiditis were the predictive factors for an ENE. A sufficient preoperative workup for LNM appears to be necessary for younger age groups, males, and tumor sizes larger than 10 mm. If LVI or ETEs are observed on pathological evaluation, closer follow-up is required to evaluate local recurrence.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: JHA, HKC. Data curation: JHA. Formal analysis: JHA. Investigation: JHA. Methodology: JHA, HKC. Project administration: JHA. Resources: JHA. Software: JHA. Supervision: HKC. Validation: JHA. Visualization: JHA. Writing - original draft: JHA. Writing - review & editing: HKC.