Comparison of the efficacy and complications of endoscopic incisional therapy and balloon dilatation for benign esophageal strictures
Article information
Abstract
Background
Benign esophageal strictures are treated endoscopically, often with balloon dilatation (BD) or bougie dilators. However, recurrent esophageal strictures have been reported after BD, and severe complications sometimes occur. The aim of this study was to compare the efficacy and complications of endoscopic incisional therapy (EIT) and BD for benign esophageal strictures.
Methods
We retrospectively reviewed patients who underwent BD or EIT as primary treatment for benign esophageal strictures between July 2014 and June 2021. Technical success was defined as restoration of the lumen diameter with <30% residual stenosis. Clinical success was defined as no recurrence of dysphagia within 1 month after BD or EIT and an increase of 1 grade or more on the Functional Oral Intake Scale.
Results
Thirty patients with benign esophageal stricture were enrolled. There were 16 patients in the BD group and 14 patients in the EIT group. No significant differences in technical and clinical success rates were found between the two groups. Furthermore, no significant differences in the re-stricture rate were observed between the groups. There was one complication in the EIT group and three complications in the BD group. Three patients who underwent BD had re-stricture and underwent EIT thereafter, and we regrouped patients who underwent EIT at least once. The clinical success rate was significantly higher in patients regrouped to the EIT group than in patients who underwent BD only.
Conclusions
EIT is not inferior to BD as the primary treatment for benign esophageal strictures, especially for recurrent cases.
Introduction
Benign esophageal strictures are a leading cause of dysphagia [1]. Dysphagia caused by esophageal stricture is a major source of morbidity as it increases the risk of pneumonia in the elderly, and it can lead to nutritional deficiencies and significantly impair quality of life [2,3]. Most benign esophageal strictures result from peptic strictures due to severe gastroesophageal reflux disease, which accounts for 70% to 80% [4]. Other causes include corrosive substance ingestion, postoperative anastomotic stricture, chemotherapy injury, and radiation injury [5].
Benign esophageal strictures are categorized by their etiology, diameter, length, and location as simple or complex, and they are usually treated endoscopically with balloon dilatation (BD) or bougie [6,7]. Although considered the primary treatment, endoscopic treatment with BD or bougie is associated with a recurrence rate of up to 83.3% [8,9]. With this treatment method, repeat procedures are required as the success rate of one procedure is low, and repeat procedures increase the risk of esophageal rupture [10].
Endoscopic incisional therapy (EIT) is reported to be effective for benign esophageal strictures [11]. If a simple incision procedure can be used to treat benign esophageal strictures and if the procedure is associated with low rates of recurrence and complications, the procedure should be considered a good first-line treatment. Several studies have reported that the rates of recurrence and complications following EIT for esophageal anastomotic strictures are low [12,13]. However, most of the studies investigated the therapeutic effect of EIT on postoperative anastomotic stenosis [14,15]. Therefore, it is necessary to evaluate the appropriateness of EIT as the primary treatment of benign esophageal stenosis of various etiologies.
In this study, we compared the efficacies and complications of EIT and BD for benign esophageal strictures of various etiologies.
Methods
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Busan Paik Hospital (IRB No. 2021-08-012-003). The written informed consent requirement was waved because researchers only accessed retrospectively a de-identified database for the purpose of analysis.
1. Study design and subjects
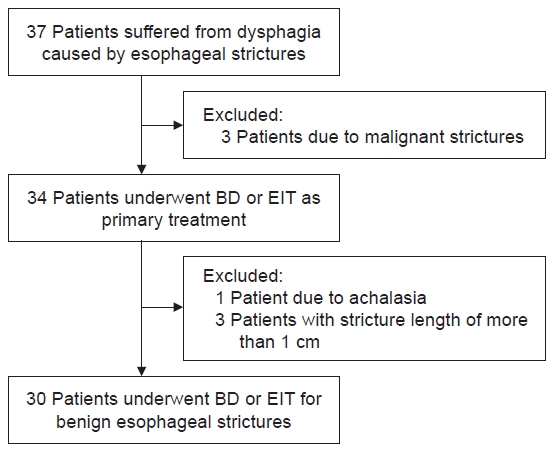
Patients who underwent BD or EIT as primary treatment for benign esophageal stricture between June 2012 and August 2021 in Busan Paik Hospital were enrolled. A total of 30 patients underwent endoscopic procedures in that period. We retrospectively reviewed the medical records of these patients for investigating their clinical characteristics. The patients with achalasia or malignant strictures were excluded. Esophageal strictures in all enrolled patients were localized and limited to less than 1 cm in length (Fig. 1). Depending on the procedure subjects underwent as first treatment, the patients were classified into a BD or EIT group. During hospitalization after the procedure, the subjects were checked for complications (e.g., esophageal perforation, bleeding, fever) and discharged if there was no abnormality in eating.
2. Definition of stricture
A stricture was defined by the inability to pass an endoscope. The degree of dysphagia was rated as a grade of one to seven on the Functional Oral Intake Scale (FOIS) [16]. Levels 1 through 3 indicate the tube dependent, and levels 4 through 7 relate to total oral intake. FOIS was defined as: level 1: no oral intake, level 2: tube dependent with minimal/inconsistent oral intake, level 3: tube supplements with consistent oral intake, level 4: total oral intake of a single consistency, level 5: total oral intake of multiple consistencies requiring special preparation, level 6: total oral intake with no special preparation, but must avoid specific foods or liquid items, and level 7: total oral intake with no restrictions. A gastroduodenoscope (GIF-H260 or GIF-HQ290; Olympus, Tokyo, Japan) with an outer diameter of 9.8 mm or 10.2 mm was used to perform the diagnosis and treatment of stricture. The diameter of the stricture was graded as >9.8 mm if the endoscope was passed freely, as 7.9–9.8 mm if a pediatric endoscope with an outer diameter of 7.9 mm (GIF-PQ260; Olympus) was passed but an adult endoscope could not be passed, and as <7.9 mm if a pediatric endoscope could not be passed.
3. Procedure assessment
Technical success was defined as restoration of lumen diameter with residual stenosis less than 30%. Clinical success was defined as no recurrence of dysphagia within 1 month after BD or EIT and an increase of one grade or more on the FOIS, which is used to determine the degree of symptom improvement the day after the procedure.
4. Endoscopic BD
Through-the-scope (TTS)-BD was performed under conscious sedation with intravenous midazolam (0.05 mg/kg) and/or propofol (0.5 mg/kg). TTS-BD catheters (Boston Scientific, Natick, MA, USA) 6–18 mm in diameter were used for 90 seconds, and the diameter of the balloon catheter was determined based on the estimated size of the stenosis. Depending on patient’s condition, ballooning size was gradually increased at intervals of 1 to 2 days and repeated several times. Ballooning was terminated when a gastroduodenoscope with an outer diameter of 9.8 mm or 10.2 mm was passed or when patient symptoms improved.
5. Endoscopic incisional therapy
All patients underwent gastroduodenoscopy under conscious sedation with intravenous midazolam (0.05 mg/kg) and/or propofol (0.5 mg/kg). An insulated-tip knife (ball endoscopic submucosal dissection knife; MTW, Wesel, Germany) was used for EIT. Incision at the stenosis site was performed at least two times (three times in most cases) and was made parallel to the longitudinal axis of the stenosis ring (Fig. 2). Mucosal incision was made using an electrosurgical generator (VIO 300D; ERBE Elektromedizin GmbH, Tübingen, Germany) and the setting was Endo Cut I mode (Effect 3, Cut duration 3, Cut interval 2). The incision of strictured mucosa was performed until the gastroduodenoscope with an outer diameter of 9.8 mm or 10.2 mm passed. The patients started taking sips of water the next morning after the EIT procedure, and if there were no symptoms, liquid diet was resumed at lunchtime on the same day.
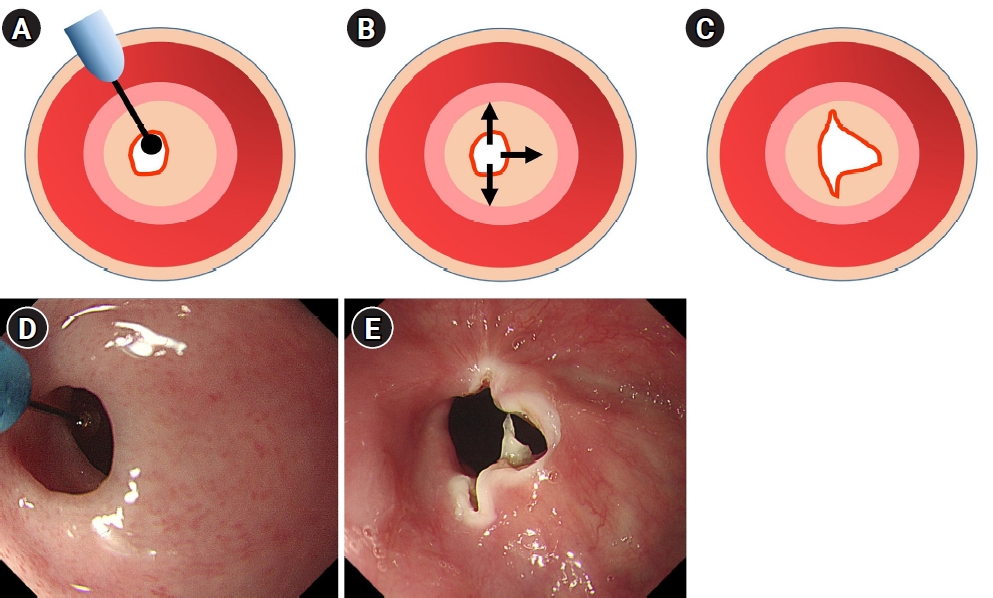
Technique of endoscopic incisional therapy for esophageal strictures. (A) Esophageal stenosis before treatment. (B) Arrows depict the radial direction of the incision. (C) Outcome of the procedure. (D) Endoscopic findings of the esophageal stricture ring. (E) Radial incisions using an insulated-tip knife, which were made parallel to the longitudinal axis of the stricture ring.
6. Statistical analysis
Descriptive data was reported as mean±standard deviation or as median and interquartile range. Differences in categorical variables were analyzed using chi-square test or Fisher exact test. Continuous variables were analyzed using the Student t-test. Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA), and p-values <0.05 were considered statistically significant.
Results
1. Patient characteristics
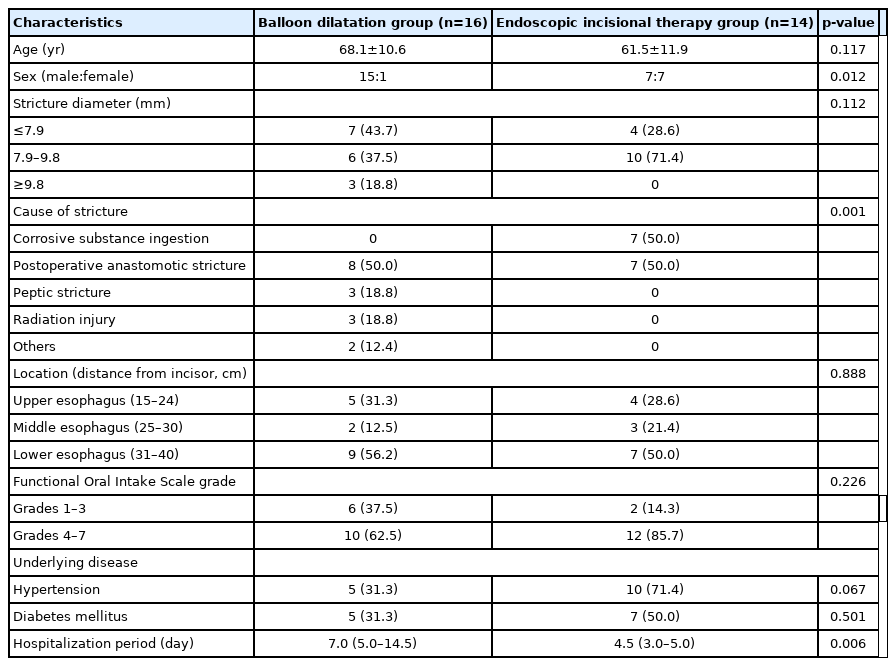
A total of 30 patients underwent treatment for benign esophageal strictures of various etiologies. Of the 30 patients, 16 underwent BD (BD group), and 14 underwent EIT (EIT group) as the first procedure. The characteristics of this study are presented in Table 1. There were significant differences in sex (p=0.012) and cause of stricture (p=0.001) between the two groups. Postoperative anastomotic stricture was the most common cause of stenosis, followed by corrosive substance ingestion (Table 1). Other causes of stenosis include peptic stricture, radiation injury, and burns. No significant differences in stricture diameter, stricture location, and FOIS grade were observed between the two groups. Based on FOIS grade, patients can be divided into a tube-dependent group (with FOIS grades 1–3) or an oral intake group (with FOIS grades 4–7). The oral intake group was the most common group in this study, making up 62.5% of the BD group and 85.7% of the EIT group. The lower esophagus was the most common stenosis location, while the middle esophagus was the least common stenosis location (Table 1). The median hospitalization periods in the BD and EIT groups were 7.0 days and 4.5 days, respectively.
2. Clinical outcomes of EIT and BD
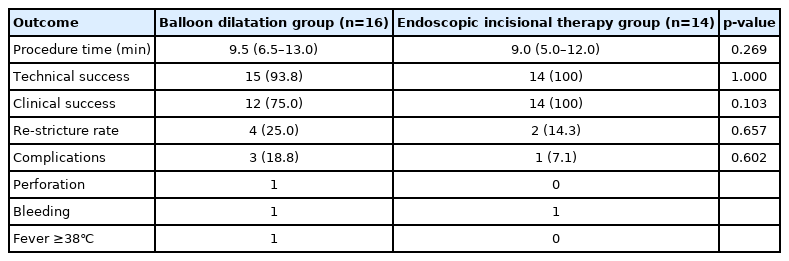
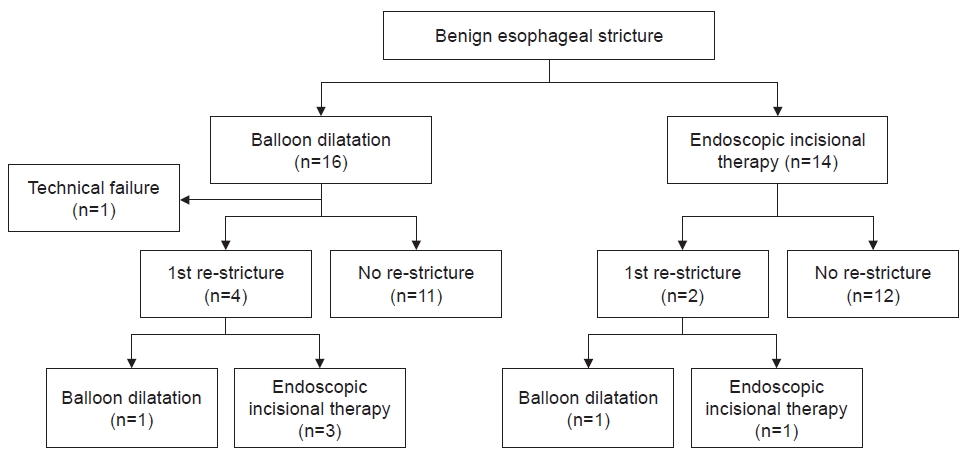
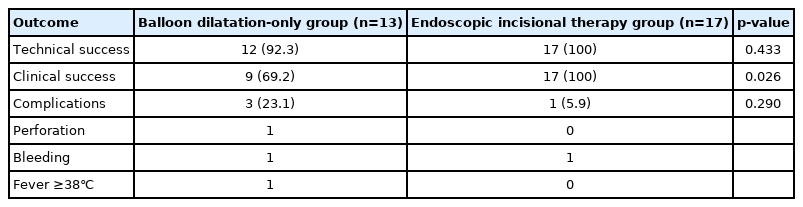
No significant differences in technical success rate and clinical success rate were observed between the EIT and BD groups (100% vs. 93.8%, p=1.000 and 100% vs. 75%, p=0.103, respectively) (Table 2). Although there was no statistically significant difference, technical success rate and clinical success rate in the EIT group were both 100%. Further, no statistically significant differences in re-stricture rate were observed between the EIT and BD groups (14.3% vs. 25%, p=0.657). In the BD group, four patients had restenosis. Of these four patients, one underwent repeat BD, while three underwent EIT. In the EIT group, two patients had restenosis. Of these two patients, one underwent repeat BD and the other underwent repeat EIT. In the BD group, re-stricture occurred in three patients within 6 months of the first procedure, and in one patient in the EIT group. There was no patient in either group who underwent BD or EIT again between 6 and 12 months after the first procedure. One re-stricture occurred in each group between 12 and 24 months. There were no statistically significant differences in the rate of complications between the two groups (p=0.602). There was one complication in the EIT group and three complications (including one case of esophageal perforation) in the BD group. The patient with esophageal perforation underwent surgery immediately after endoscopy. The median procedure time was 9.0 minutes for EIT and 9.5 minutes for BD (p=0.269). To analyze the efficacy of EIT more accurately, we regrouped patients who underwent EIT at least once, given that three patients underwent EIT after BD (Fig. 3). Clinical success rate was significantly higher in the regrouped patients who underwent EIT than in patients who underwent BD only (100% vs. 69.2%, p=0.026) (Table 3). Furthermore, the technical success rate in the regrouped patients who underwent EIT was also 100%.
Discussion
We analyzed the technical and clinical successes of BD and EIT to find out their differences. As earlier mentioned, treatment of benign esophageal strictures often requires repeat procedures [7,17], so it makes sense to reclassify patients who underwent EIT at least once. In addition, clinical success may be more suitable for evaluating treatment efficacy, as the main purpose of treatment for esophageal strictures is to improve symptoms mainly in patients with dysphagia. Therefore, we regrouped these patients, and as earlier described, we observed a statistically significant difference in clinical success (p=0.026). This means that patients who had undergone multiple BD procedures may later undergo EIT for symptom relief.
In this study, there was one case of esophageal perforation in the BD group and no case in the EIT group. A 63-year-old female with a history of rheumatoid arthritis underwent BD due to peptic esophageal stricture at lower esophagus. After BD, she complained of abdominal pain and free air was found on abdominal X-ray, so she underwent surgery. Although the incidence rate of esophageal perforation is not high (0.37%–4.9%), the condition can be fatal [18,19]. The case of esophageal perforation in the BD group is thought to be because the weak part of the esophageal wall can easily be perforated even when the pressure applied to it is the same as that applied to other parts of the esophageal wall during BD. In contrast, EIT is associated with a relatively low risk of esophageal perforation if the depth of incision is appropriate and no pressure is applied. In previous studies, it was reported that electrocautery incision is safe and is associated with a low rate of complications, including perforation [20,21]. Thus, EIT is considered a safer therapeutic option for benign esophageal stricture than BD.
Regarding EIT, it is necessary to discuss the appropriate number and depth of incisions. We performed incision about three times before the endoscope, which has an outer diameter of 9.8 mm, could pass through. Usually, an average of eight to 12 incisions are needed [12], and the depth of incision is evaluated based on the length of the needle-knife [11]. However, the number and depth of incisions may vary depending on the etiology and location of the stenosis and on the condition of the patient. A study on depth measurement using ultrasound and on the number of incisions would be beneficial.
In this study, the most common cause of benign esophageal stricture was postoperative anastomotic stricture (15 cases), followed by corrosive substance ingestion (7 cases). In general, esophageal stricture due to gastroesophageal reflux disease is considered the most common type of esophageal stricture, but its incidence has been decreasing since the 1990s likely due to the widespread screening endoscopes and increase in the use of proton pump inhibitors [22]. A previous study compared the efficacies of EIT and BD for postoperative esophageal strictures [23]. However, there are no studies on the efficacy of EIT for corrosive esophageal strictures. Although BD is often considered the primary treatment option for corrosive esophageal strictures, it is associated with a high recurrence rate and poor outcomes in the chronic stage [24], and surgical treatment is sometimes effective [25]. In our study, there were seven patients with corrosive esophageal stricture, and since all the patients underwent EIT, it was not possible to directly compare the efficacies of EIT and BD for corrosive esophageal stricture. It is thought that EIT was chosen for all seven patients because the efficacy of BD would be low given that patients with corrosive esophageal stricture typically present at hospitals in the chronic stage of the disease.
This study has several limitations. First, the number of patients was so small that it was difficult to determine statistical significance. As with multicenter studies, it is necessary to conduct large-scale studies with a high number of patients. Second, during the follow-up period of this study, there were no confirmed cases of recurrence of stenosis, but there may be recurrence of stenosis in the future. Therefore, it is necessary to follow up on the patients over the long term. Third, due to differences in the etiology of stricture between the two groups, objective comparison of the efficacies of EIT and BD was difficult. To correct for these differences, propensity-score matching was attempted; however, the attempt was unsuccessful due to the small number of patients.
With advancements in endoscopic techniques, endoscopic treatments (e.g., per-oral endoscopic myotomy), which are not inferior to surgery, are increasingly attempted [26]. However, no matter how advanced the endoscopic technology is, if repeat procedures are required, patient compliance may decrease and lead to serious complications. EIT was introduced to overcome the drawbacks of BD. Our study results support those of previous studies, especially regarding the efficacy of EIT for esophageal strictures of various etiologies.
In conclusion, EIT is not inferior to BD as primary treatment for benign esophageal stricture. Especially, for recurrent benign esophageal strictures, it may be more effective than BD.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: HSL. Data curation: SHL, HWB. Formal analysis: EJC, JSY. Investigation: EJC, SJY. Methodology: SRJ, JHK. Project administration: HSL. Supervision: SRJ. Visualization: JHH. Writing - original draft: EJC. Writing - review & editing: HSL. Approval of final manuscript: all authors.