Sigmoid colon plexiform neurofibroma as a colonic subepithelial mass: a case report
Article information
Abstract
Plexiform neurofibroma (PN) is an uncommon benign tumor, usually associated with neurofibromatosis type 1. As most PNs involve the craniomaxillofacial region, PN of the colon is very rare. Here we present a case of PN involving the sigmoid colon. A 43-year-old male patient presented to the outpatient clinic for the evaluation of an incidentally discovered sigmoid colon mass. A colonoscopic biopsy was performed for the mass, and the result revealed neuronal proliferation. The patient visited the outpatient clinic a year later with symptoms of abdominal pain and stool caliber change. Biopsy was repeated for the sigmoid colon mass, and the results showed mucosal Schwann cell proliferation and S-100 immunostaining positivity. Computed tomography and magnetic resonance imaging were performed for further evaluation, and neurofibroma or schwannoma was suspected based on the imaging studies. For an accurate diagnosis, the patient underwent surgery to remove the sigmoid colon mass. The final diagnosis of the mass was confirmed as PN. We hereby report a rare case of PN involving the sigmoid colon that could not be diagnosed before surgery.
Introduction
Plexiform neurofibroma (PN) is a benign tumor that involves the peripheral nerve sheath and is most commonly associated with neurofibromatosis type 1 (NF-1) [1]. NF-1 is a neurocutaneous disease with an incidence of 1 in 3,000 births. The NF-1 gene (NF-1) is located on chromosome 17q11.2, and NF-1 is caused by a loss-of-function mutation of this gene. NF-1 produces the neurofibromin protein, a member of the Ras GTPase-activating protein family [2]. There are four types of neurofibromas, namely; cutaneous neurofibromas, subcutaneous neurofibromas, nodular PN, and diffuse PN [3]. PNs are uncommon variants of NF-1 that occur most frequently in the craniomaxillofacial region [4]. In cases of PN involving the gastrointestinal tract, the most commonly affected sites are the jejunum and stomach [5]. Accordingly, PNs affecting the colon are rarely reported. Herein, we present a case of PN that involved the sigmoid colon.
Case
Ethical statements: This study was exempted from review by the Institutional Review Board (IRB) of Inje University Busan Paik Hospital (IRB No. BPIRB 2022-03-001). The informed consent was waived for this case study.
A 43-year-old man presented to the gastroenterologist for the evaluation of a sigmoid colon mass. The patient had no medical history; however, his mother had a history of NF-1. He had previously undergone colonoscopy at a local clinic for health screening, with a sigmoid colon mass identified despite the absence of symptoms (Fig. 1A). A colonoscopic biopsy of the sigmoid colon mass was performed at local clinic, with histological analyses demonstrating neuronal proliferation. As the patient had no symptoms, he was only expected to undergo a follow-up colonoscopy after 1 year.
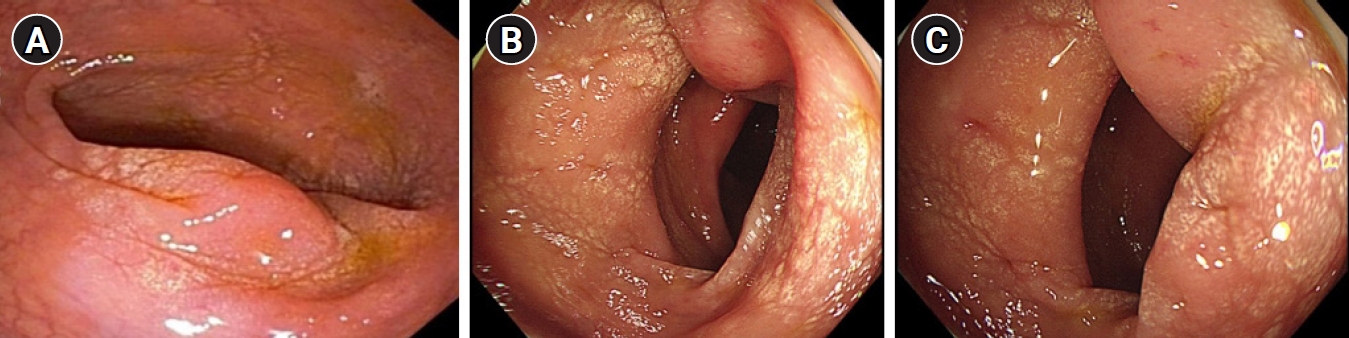
Colonoscopic findings. (A) An encircling, edematous, colonic mass at the sigmoid colon was observed on initial colonoscopy. (B, C) An encircling, edematous, mucosal lesion at 23 cm from the anal verge on colonoscopy at the time of a follow-up evaluation with symptoms 1 year later. There were no significant changes in the findings of the follow-up colonoscopy compared to the initial colonoscopic findings 1 year prior.
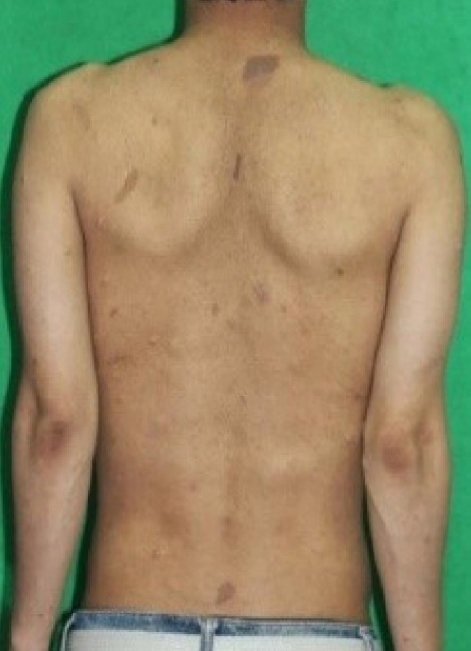
A year later, the patient visited the outpatient clinic in our hospital with symptoms of stool caliber change and abdominal discomfort. Initial vital signs were stable with a blood pressure of 140/90 mmHg and a pulse rate of 95 beats per minute. All the initial laboratory test results performed in our hospital were within the normal range. Colonoscopy was also performed, which showed an encircling edematous mucosa lesion at the sigmoid colon (Fig. 1B, 1C). Therefore, a repeat of the biopsy for the sigmoid colon mass was performed. The result presented as chronic colitis with mucosal Schwann cell proliferation, and the immunostain of S-100 was positive (Fig. 2). Since the colonoscopic biopsy was performed only in the mucosal layer, submucosal invasion could not be confirmed. We then performed abdominal computed tomography (CT) scan for further evaluation. The abdominal CT scan revealed annular wall thickening and multinodular soft tissue mass on the mesosigmoid about 6 cm in long diameter (Fig. 3A). Neurogenic tumor was suspected, and the patient underwent pelvis magnetic resonance imaging (MRI) upon recommendation. The findings of pelvis MRI were similar to those of the CT scan, where MRI results suggested that the mass might be a neurogenic tumor, such as mesenteric neurofibroma or schwannoma (Fig. 3B). For accurate diagnosis and since the patient showed symptoms, the patient underwent a laparoscopic anterior resection of the sigmoid colon. The final diagnosis was reached by pathology analysis following laparoscopic surgery with complete resection, which showed that the tumor was PN (Fig. 4). We did not perform endoscopic ultrasonography as a large sigmoid colon mass was clearly revealed on abdominal CT scan, and surgery was planned for accurate diagnosis. Furthermore, we requested the neurology department to genotype the neurofibromatosis. DNA sequencing of blood sample was performed, and a heterozygotic NF-1 mutation was detected. In addition, the patient was diagnosed as having NF-1 as he satisfied the diagnostic criteria, which were the presence of six or more café-au-lait macules (Fig. 5), the presence of one PN and a first-degree relative with NF-1. The patient was discharged approximately 2 weeks after the surgery without postoperative complications, and has been presented to the outpatient clinic for follow-ups.
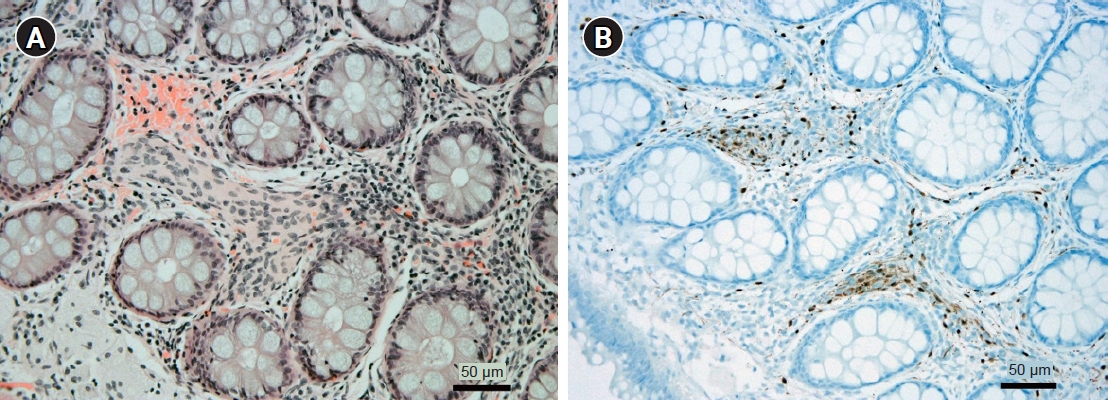
Pathologic findings of the colonoscopic biopsy. (A) Spindle cells with plump cytoplasm were noted in the lamina propria (hematoxylin and eosin stain, ×200). (B) The cells were positive for S100 protein (anti-S100 immunohistochemical stain, ×200).

Imaging findings. (A) Computed tomography was performed for further evaluation, demonstrating annular wall thickening and a 6-cm multinodular soft tissue mass in the mesosigmoid colon (arrow). (B) Magnetic resonance imaging of the pelvis demonstrating a mesosigmoid mass. There was an irregular, lobulated, 6.2-cm-diameter, T2-high-intensity lesion at the mesosigmoid with adjacent focal annular wall thickening at the mid-sigmoid colon (arrow).

Image of the resected specimen and the pathologic findings of a sigmoid colon mass after complete surgical removal. (A) The resected segment of the colon was approximately 6.5 cm in length and 4.2 cm in circumference (arrow). (B) Lower-power view demonstrating multiple well-defined nerve bundles with nodular appearance (hematoxylin and eosin stain, ×10). (C) High-power view of representative area of fibroblast-like cells scattered in wire-like collagen fibrils. No nuclear palisading or Verocay bodies were observed (hematoxylin and eosin stain, ×40).
Discussion
Neurofibromatosis is a generalized disorder that can be classified into the following two types: (1) NF-1, arising from Schwann cells; and (2) central type 2, which mostly involves the central nervous system [1]. NF-1 is more common than type 2 neurofibromatosis and accounts for most cases of neurofibromatosis [1]. For NF-1 to be diagnosed, two of the following diagnostic criteria should be satisfied: (1) six or more café-au-lait macules; (2) two or more neurofibromas of any type or one PN; (3) freckling in the axillary or inguinal region; (4) optic glioma; (5) two or more iris Lisch nodules (iris hamartomas); (6) distinctive bony lesions, such as pseudoarthrosis; and (7) a first-degree relative with NF-1 [6].
Intra-abdominal neoplasms associated with NF-1 can be classified into five categories. The first category is neurogenic tumors, which is the most common type of neoplasm associated with NF-1, and PN is included in this category [7]. The second category is a gastrointestinal stromal tumor (GIST) [7]. The third category is neuroendocrine tumors, such as pheochromocytoma and carcinoids [7]. The fourth category is embryonal tumors, which include neuroblastoma and rhabdomyosarcoma [8]. The last category is adenocarcinomas, which involve the gastrointestinal tract [8].
PN is a rare subtype of benign nerve sheath tumors, termed neurofibromas. PN accounts for up to 30% of cases of NF-1. PN can involve the skin and peripheral nerves of internal organs. However, PN most frequently involves the craniomaxillofacial region. Therefore, PN involving the gastrointestinal tract appears to be rare with only a small number of cases reported (Table 1) [1,9-13]. PN involves multiple fascicles of peripheral nerves and may involve multiple branches of large nerve trunks [14]. PN develops from Schwann cells of the peripheral nervous system and is frequently observed in cases of NF-1. Symptoms are generally nonspecific and usually related to tumor mass effects, such as a palpable abdominal mass, gastrointestinal tract obstruction, and jaundice. Bleeding can also occur if the mucosa is involved [15]. Therefore, the accurate diagnosis of PN is clinically challenging based on physical examination and clinical symptoms alone without imaging tests such as CT or MRI. PN usually presents as homogenous and hypodense masses on enhanced CT images [14]. MRI is the standard modality for the evaluation of tumors of neural origin. PN has been described as being characteristically lobulated with a hyperintense signal on T2-weighted imaging and hypointense on T1-weighted imaging [16]. Colonic neurofibromas detected on endoscopy are usually observed as sessile or pedunculated mucosal or submucosal lesions, with a size ranging from a few millimeters to several centimeters [17].
It is important to differentiate neurofibromas from other spindle cell soft tissue tumors, particularly GISTs, and immunostaining plays an important role in the diagnosis of neurofibroma. GISTs demonstrate expression of S-100 and CD34, similar to neurofibroma; however, neurofibromas typically have strong expression of CD117 and DOG1 [17]. Pathologically, PN involves multiple large nerve trunks with irregular arrangements [18]. Moreover, PN is typically surrounded by cellular matrices that contain spindle- and S-shaped nuclei, strands of collagen, mucin, and mast cells [18].
Patients with NF-1 have a 50% risk of passing on the disease to offspring, and NF-1 mutation is usually found in approximately 85% to 95% of patients with NF-1 using a combination of molecular techniques [19]. Prenatal testing to detect mutations in NF-1 is possible through amniocentesis or chorionic villus sampling [19]. Genetic counseling before fertilization is recommended for all patients with NF-1 [19].
PN undergoes a malignant transformation with the risk of developing malignant peripheral nerve sheath tumors in patients with PN increased by approximately 20-fold [20]. Therefore, complete surgical resection of PNs is advocated when surgery represents an appropriate option. However, PN is technically difficult to completely resect if the tumor invades surrounding tissues [14]. When surgery is not possible, chemotherapy may represent an alternative treatment option for PN [20]. Several ongoing studies are evaluating the efficacy of biological agents for the treatment of PN [20].
In this report, we present the rare case of a patient with PN involved in the sigmoid colon that was found as subepithelial mass. After surgery, the patient was diagnosed with PN. Although colonic involvement of PN is uncommon, the possibility of PN should be considered when colon mass is found in patients with a family history of NF-1. As PN has the potential to become malignant, if there is a possibility of PN, diagnosis and treatment through surgery as well as endoscopy and radiology examinations should be considered. As colonic involvement is rare in patients with PN, there is no established guideline for the management of patients with colonic PN after surgery. However, follow-up assessments using endoscopy and imaging study after surgery for PN should be performed considering the high incidence of malignancies and recurrence.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: HWB, HSL. Data curation: HWB, SJY, HSL. Formal analysis: HWB, HSL, SRJ. Investigation: HWB, HB, HSL. Methodology: HWB, EJC, MK, HSL. Project administration: HWB, HSL. Resources: HWB, HSL, SHL. Supervision: HWB, HSL. Validation: HWB, HSL. Visualization: HWB, HSL. Writing - original draft: HWB, HSL. Writing - review & editing: HWB, HSL. Approval of final manuscript: all authors.