Development of severe junctional bradycardia after dexmedetomidine infusion in a polypharmacy patient: a case report and literature review
Article information
Abstract
The authors report a case of newly manifested severe junctional bradycardia following dexmedetomidine administration during spinal anesthesia in a polypharmacy patient. A 77-year-old woman receiving multiple medications, including a beta-blocker and a calcium channel blocker, underwent right total knee arthroplasty. After spinal anesthesia, intravenous dexmedetomidine was initiated as a sedative; her heart rate decreased, followed by junctional bradycardia (heart rate, 37–41 beats/min). Dexmedetomidine was discontinued, and a dopamine infusion was initiated. Seven hours after surgery, junctional bradycardia persisted; a temporary transvenous pacemaker was inserted, and the beta-blocker and calcium channel blocker were discontinued. The patient was discharged on postoperative day 11 without any sequelae. Anesthesiologists should be aware of dexmedetomidine’s inhibitory effects on the cardiac conduction system, especially in geriatric patients taking medications with negative chronotropic effects and in combination with neuraxial anesthesia.
Introduction
Dexmedetomidine, an alpha-2 adrenergic agonist [1], has generally been considered a relatively safe sedative agent, rarely resulting in hypotension and respiratory depression [2]. However, several previous studies have reported that dexmedetomidine has a negative chronotropic effect on the sinoatrial node, leading to severe bradycardia and even sinus pause or arrest [3,4]. Life-threatening bradyarrhythmia is typically related to the co-administration of dexmedetomidine with other drugs rather than dexmedetomidine alone [3,4]. The global increase in aging populations corresponds with the gradual increase in the proportion of surgical patients with multimorbidities and polypharmacy [5]. In this context, it is necessary to consider the patients’ preoperative medication history in selecting the appropriate sedative agents and clinical applications. In this article, we report a case involving a polypharmacy patient with newly manifested severe junctional bradycardia following dexmedetomidine administration during spinal anesthesia.
Case
This case report was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2111-008-108). Written informed consent was obtained from the patient.
A 77-year-old woman (153 cm, 60 kg) was admitted for right total knee arthroplasty. The patient was diagnosed with chronic hepatitis C, asthma, hypertension, and intermediate coronary artery occlusive disease (stenosis diameter ≥40% but ≤70%). Preoperative laboratory and chest radiography findings were unremarkable. However, an electrocardiogram (ECG) revealed sinus bradycardia (50 beats/min) (Fig. 1A). Preoperative transthoracic echocardiogram demonstrated normal left ventricular systolic function (ejection fraction 60%), and no evidence of regional wall motion abnormalities. The patient took medicines with furosemide (20 mg/day), bisoprolol (2.5 mg/day), fimasartan (120 mg/day), diltiazem (360 mg/day), isosorbide mononitrate (40 mg/day), rosuvastatin (5 mg/day), rabeprazole (10 mg/day), and umeclidinium bromide/vilanterol inhaler. The cardiologist advised taking all medications immediately before surgery.
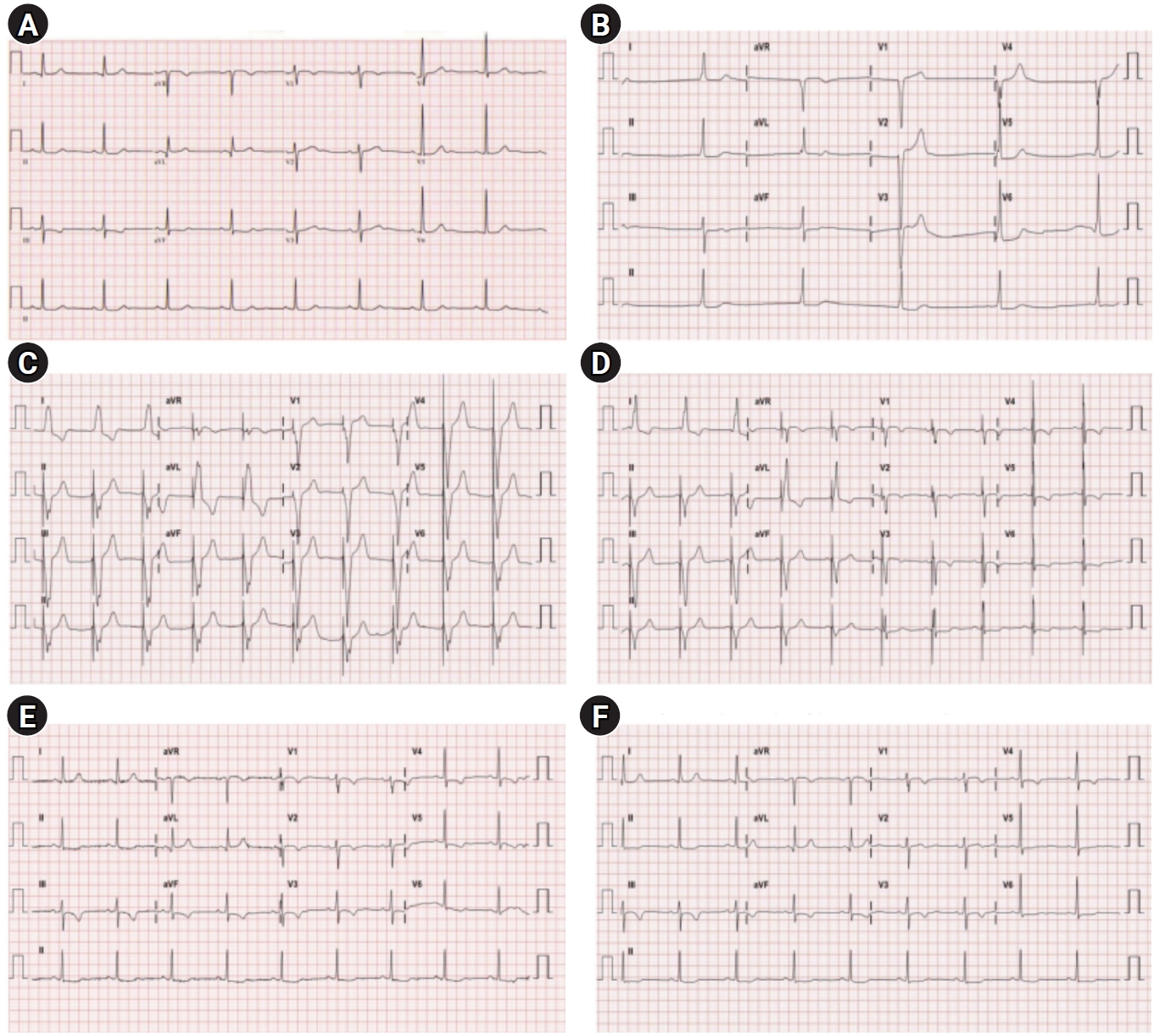
Electrocardiogram. (A) Pre-operation (HR 50 beats/min). (B) Operative day (HR 30 beats/min). (C) POD 1 (HR 60 beats/min; temporary pacemaker inserted). (D) POD 2 (HR 60 beats/min; temporary pacemaker inserted). (E) POD 3 (HR 55 beats/min; temporary pacemaker inserted). (F) POD 4 (HR 53 beats/min; temporary pacemaker inserted). HR, heart rate; POD, postoperative day.
The patient’s baseline (preoperative) heart rate (HR), oxygen saturation (SpO2), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were 47–56 beats/min, 96%–98% (room air), 100–140 mmHg, and 70–90 mmHg, respectively.
Vital signs and drugs used during surgery are reported in Fig. 2. For spinal anesthesia, 3 mL of 0.5% bupivacaine hydrochloride in dextrose was injected into the subarachnoid space using a 23-gauge spinal needle at the L3/4 level. After 10 minutes of spinal anesthesia, the sensory blockade level, as determined by cold sense with an alcohol swab, was up to T8; no further increase in the sensory blockade level was observed. Subsequently, ultrasound-guided femoral nerve catheterization was performed (Fig. 2). As per the standard regimen, dexmedetomidine was administered intravenously with a bolus loading dose of 1 μg/kg for 10 minutes, followed by a maintenance dose of 0.8 μg/kg/hr for 5 minutes. During the loading dose infusion of dexmedetomidine, a decrease in HR was observed (36 beats/min), and 500 μg of atropine was administered intravenously. The sedation level (Richmond Agitation Sedation Scale, –2; briefly awakens with eye contact to voice) and blood pressure (SBP: 100–107 mmHg, DBP: 50–50 mmHg) remained stable; however, junctional rhythm with hidden p waves was observed, and severe bradycardia (HR, 37–41 beats/min) persisted. Dexmedetomidine was discontinued. Subsequently, as there was no definitive finding of acute myocardial ischemia on electrocardiogram, intravenous dopamine infusion was initiated (5–10 μg/kg/min). Afterward, during surgery, the patient’s vital signs remained stable, except for mild to moderate bradycardia (HR, 42–55 beats/min). At 30 minutes before the end of the operation, severe bradycardia and junctional rhythm (33 beats/min) recurred, and dobutamine infusion (5–10 μg/kg/min) was administered. However, HR reactivity to dobutamine was not observed, and dobutamine infusion was terminated.

Vital signs and drugs used during the operation. Drugs and events: (1) spinal anesthesia was performed, (2) femoral nerve catheterization was performed and dexmedetomidine administration was started, (3) atropine (500 μg, intravenously), (4) junctional rhythm with hidden P waves was observed, (5) dopamine infusion was initiated and dexmedetomidine was discontinued, (6) severe bradycardia and junctional rhythm recurred and a dobutamine infusion was initiated, (7) the dobutamine infusion was terminated. HR, heart rate; MBP, mean blood pressure; SpO2, oxygen saturation; RR, respiratory rate.
In the recovery room, dopamine infusion and close monitoring continued. The patient’s vital signs were as follows: HR, 28–51 beats/min; SpO2, 93%–97% (room air); SBP, 111–168 mmHg; DBP, 52–82 mmHg; and respiratory rate, 18–20 breaths/min. Seven hours after administration of spinal anesthetic, the patient was alert and the sensory blockade level dropped below S1, although junctional bradycardia with hidden p waves persisted (Fig. 1B). The levels of cardiac biomarkers were within the normal range. The patient was transferred to an intensive care unit, a cardiologist was consulted, and a transvenous temporary pacemaker via a left femoral vein (VVI mode) was inserted (Fig. 1C and D). Bisoprolol and diltiazem were discontinued. The patient was transferred to the general ward on a postoperative day (POD) 3. The patient’s intrinsic sinus rhythm was restored on POD 3 (Fig. 1E), and the temporary pacemaker was removed on POD 4 (Fig. 1F). The patient was discharged on POD 11 without any sequelae.
Discussion
Although dexmedetomidine has been considered to have minimal effects on the circulatory and respiratory systems in clinical settings [2], cases of dexmedetomidine-induced severe bradycardia leading to cardiac arrest have also been reported [3,4]. These fatal complications are particularly prone to occur when dexmedetomidine is administered in patients with multiple risk factors [3,4]. In the present case, the presumed risk factors for dexmedetomidine-related bradycardia were combined neuraxial block [6], patient’s age [7], and multiple negative chronotropes [3].
Spinal anesthesia can also cause bradycardia, with a reported incidence of 10% to 15%, although, in most patients, significant changes in HR are not observed [8]. The presumed mechanisms of bradycardia following spinal anesthesia include a block of sympathetic cardioaccelerators arising from T1 to T4 segments and a decrease in venous return and filling pressure [8,9]. In addition, Hong et al. [6] reported that concomitant use of dexmedetomidine as sedation during spinal anesthesia improves postoperative analgesia but increases the risk for bradycardia.
Advanced age is a risk factor for dexmedetomidine-related bradycardia [7], and many geriatric patients are exposed to polypharmacy. In particular, it has been reported that the prevalence of polypharmacy in hypertension, angina, and congestive heart failure, diseases related to the prescription of negative chronotropes, reached 51%, 42%, and 60%, respectively [5]. These results suggest that, before administering dexmedetomidine to elderly patients, the medication history covering both currently and recently prescribed drugs should be fully considered. In this case, the risk factors for junctional dysrhythmia among the patient’s medications are bisoprolol and diltiazem, for which negative chronotropic effects have been reported [10].
The advantages of dexmedetomidine for reducing postoperative delirium, postoperative pain burden, and postoperative nausea and vomiting have been consistently demonstrated in patients who undergo total knee arthroplasty [11,12]; as such, the popularity of dexmedetomidine as a sedative agent is predicted to increase in these population. However, in this case, it was evident that the patient had multiple risk factors for developing cardiac conduction disorder with dexmedetomidine; the patient was elderly, underwent spinal anesthesia, and took various negative chronotropes. Therefore, it is strongly suspected that there was an additive or synergistic interaction between dexmedetomidine and these risk factors, resulting in cardiac conduction disturbances leading to the junctional rhythm. In this case, the patient was also administered the usual dose of dexmedetomidine for procedural sedation. Unfortunately, there is no consensus on the safe and tolerated dose of dexmedetomidine in the high-risk population [13,14]. However, previous clinical trials have pointed out that aging and regional anesthesia reduce the amount of dexmedetomidine required for sedation [13,14]. In addition, the elimination half-life of dexmedetomidine, reported to be about 2.1–3.1 hours, is markedly prolonged with aging [14,15]. Thus, a reduction of dexmedetomidine administration should be considered in patients at high risk of dexmedetomidine-induced cardiac conduction disorder.
The management guidelines for acute symptomatic junctional dysrhythmia, associated with sinoatrial node dysfunction, are as follows [10,16]: (1) preferentially, 0.5–1.0 mg of atropine is administered as a bolus intravenously, except for patients who have undergone heart transplantation; (2) in case of hemodynamically unstable, acute temporary cardiac pacing should be performed; (3) without definitive findings of acute myocardial ischemia, administration of beta-agonists such as isoproterenol, dopamine, dobutamine, or epinephrine could be considered to increase HR and improve symptoms; or (4) as in this case, when junctional arrhythmia due to calcium channel blocker or beta-blocker overdose is suspected, calcium or glucagon could be additionally considered an antidote.
Compared with previously reported cases, the present case had the following features: the patient was not in critical condition and without substantial functional limitations; no other sedative or analgesic agents other than dexmedetomidine were used concomitantly, and all negative chronotropic agents concomitantly used were daily medications taken before surgery. In this context, the present case is a more general condition and common scenario encountered in the field of anesthesia; however, the incidence of dexmedetomidine-associated severe bradycardia has been considered rare. In this regard, Ohmori et al. [17] suggested that some cases of dexmedetomidine-induced cardiac conduction disorders may have been overlooked.
In summary, we report a case of severe junctional bradycardia in a patient receiving a dexmedetomidine infusion during spinal anesthesia. Close monitoring and effective treatment of this adverse event resulted in complete recovery without complications. Nevertheless, anesthesiologists should pay attention to the inhibitory effects of dexmedetomidine on the cardiac conduction system, especially in the geriatric population and in patients taking medications with negative chronotropic effects and in combination with neuraxial anesthesia.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This work was supported by a clinical research grant from Pusan National University Hospital in 2020.
Author contributions
Conceptualization: SJ, EK, SHL. Data curation: SJ, SHL, SIP. Funding acquisition: EK. Project administration: SJ, EK. Supervision: EK. Visualization: SIP, HSR. Writing - original draft: SJ, SIP, HSR, DL. Writing - review & editing: SJ, EK, DL.
