Articles
- Page Path
- HOME > Kosin Med J > Volume 33(1); 2018 > Article
-
Case Report
Pontine Hypoplasia and Cri-du-chat Syndrome in a Preterm Infant - Yu Jin Jung
-
Kosin Medical Journal 2018;33(1):117-121.
DOI: https://doi.org/10.7180/kmj.2018.33.1.117
Published online: June 30, 2018
Department of Pediatrics, College of Medicine, Kosin University, Busan, Korea.
- Corresponding Author: Yu Jin Jung, Department of Pediatrics, College of Medicine, Kosin University, 262, Gamchen-ro, Seo-gu, Busan 49267, Korea. Tel: +82-51-990-3336, Fax: +82-51-990-3071, hasaohjung@hanmail.net
• Received: October 21, 2016 • Revised: January 4, 2017 • Accepted: January 16, 2017
Copyright © 2018 Kosin University College of Medicine
- 983 Views
- 5 Download
- 1 Crossref
Abstract
- A premature infant with gestational age 36+4 weeks was admitted with respiratory distress syndrome. Surfactant and ventilation were firstly done to improve his respiration. After extubation, weak, high-pitched cry and asymmetric face with micrognathia and hypertelorism were detected. Therefore, cytogenetic analysis was performed, and his karyotype was 46, XY, del(5) (p14p15.33). Pontine hypoplasia was detected on cranial magnetic resonance imaging (MRI). Therefore, karyotyping and cranial MRI should be performed in case of preterm infants with suspicion of Cri-du-chat syndrome (CdCS).
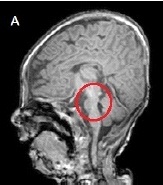
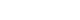
- A male infant was admitted to the neonatal intensive care unit for respiratory distress syndrome. He was born at 36+4 weeks, with birth weight of 1,720 g through emergency cesarean delivery because of fetal distress. There was no family history of genetic disease. Oligohydramnios and intrauterine growth retardation were noticed from week 5 of pregnancy. The Apgar score was 4 points at 1 minute and 7 points at 5 minutes. A birth weight of 1,720 g (< 10th percentile), a length of 41 cm (< 10th percentile), and microcephaly with a head circumference of 30.5 cm (< 10th percentile) at birth were below the 10th percentile. Grunting, respiratory difficulty, and chest retraction were observed in the first few minutes after birth. Tracheal intubation was performed due to progressive respiratory failure. Ventilator treatment was started and pulmonary surfactant was administered through an endotracheal tube. Facial dysmorphism with severe asymmetry and increased distance between the eyes were distinctive on the physical examination at birth. But his cry was not noticed because of the ventilator. After improvement of the respiratory symptoms and chest radiography at 3 days after birth, the infant was extubated, On re-examination, his chin was found to be small and the head was tilted to the left. Thereafter, the infant could not easily suck from the bottle, showed feeding hypoxemia and decreased muscle tone. All laboratory studies including TORCH (Toxoplasma, Rubella, Cytomegalovirus, Herpes simplex virus) which had been performed to identify the cause of intrauterine growth retardation and congenital metabolic panel tests were normal. The infant's cry observed after extubation was weak and regarded as just one of the premature infant's abnormal cries post-intubation. But the cry gradually became high-pitched and sounded like a cat's cry. Also, several distinctions in his face became clear: the eyes sagged with canthal wrinkles, the nose had a low ridge, and microcephaly was observed. In addition to these evaluations, renal ultrasound, echocardiogram, and hearing test were performed to check for any other abnormality or associated diseases. A slightly small sized kidney and a 5 mm-sized atrial septal defect with a septal aneurysm were observed in this patient. Also, the hearing test result such as an automated auditory brainstem response was normal. Therefore, additional evaluations were performed through brain magnetic resonance imaging (MRI) and chromosome analysis. Through MRI on the 17th day after birth, pontine hypoplasia with no other abnormality was identified (Fig. 1). The CdCS was diagnosed through chromosome analysis of 46, XY, del (5) (p14p15.33). Also, a specific deletion in the region from 5p15.33 to 5p14.2 was identified by chromosomal microarray analysis. The infant patient was discharged on the 75th day after birth when he was well enough to be cared in a home setting after bottle-feeding practice and rehabilitation. At the age of 5 months, he was 58 cm tall and weighed 4.3 kg, which were less than 3 percentile compared to those of the same age. On Korean Bayley infant development examination, the cognitive, language, and motor scores of the infant were similar to/like those of a two months old infant. He has also been enrolled in a rehabilitation physical therapy program three times a week to cure the worsening neck and body tilting problem, which made it difficult for him to control his neck.
CASE
- The Cri du chat syndrome (CdCS) is a genetic disease caused by deletion of the short arm of chromosome 5. This syndrome was first reported by Lejeune et al. in 1963 in terms of its clinical and cytogenetic aspects.4 The deletion regions range from total loss of the short arm of chromosome 5 to a partial deletion from 5p13 to 5p15.56 The size of the deletion ranges from 5Mb to 40Mb. The incidence of the CdCS in live births is 1:15,000~1:50,000,56 and the morbidity rate in 6,000 mentally retarded infants is about 1 in 350.7 Although there is a slightly higher incidence in girls,47 there are no racial or geographic differences.5
- CdCS is characterized by the facial appearance with ocular hypertelorism and the presence of epicanthal folds.5 Although intubation partly hindered closer observation of the infant's face at birth, severe facial asymmetry, small chin, and head tilted to the left were barely observed. Also the infant's first cry after extubation was weak, which was then considered to be caused by intubation or as one of the premature infant's cries. Along with the difficulty in bottle feeding, the change from a weak cry to a cat like high-pitched cry aroused suspicion that the cause of the abnormal crying sound arose from the CdCS. Therefore, constant concern about physical changes and the expression of new symptoms is crucial considering that the diagnosis of a chromosomal anomaly like cat-cry can be delayed in case of a premature infant with respiratory difficulty or under intensive care for acute diseases at birth. Moreover, these characteristic cries disappear within a few months after birth or by one year of age.5 Hence, they are not found in all patients6 and they may be observed less frequently among older infant patients.58 Therefore, high-pitched cries are not the only clue for the CdCS unless the patients are in the early days after birth such as the case of this infant.
- Neuroradiological procedures such as cranial MRI can provide a critical clue in confirming the CdCS in the early period of life in neonates or preterm infants who cannot easily or clearly be diagnosed as having this syndrome. Other clinical characteristics of CdCS can be observed in the neuroradiological findings, one of which is known to cause the existence of hypoplasia of the brainstem, particularly in pons and cerebellum.9 Generally, pontine hypoplasia is demonstrated in heterogeneous diseases such as pontocerebellar hypoplasia, congenital disorder of glycosylation, congenital muscular dystrophy, and cerebellar artery rupture in preterm infants.10 However, it has been reported that this pontine hypoplasia can be an important clue to diagnose the CdCS, because CdCS is actually one of the causes of brainstem hypoplasia predominantly at the pontine level.9 Thus, the possibility of CdCS should be considered if brainstem hypoplasia is found, especially in the pons, on cranial MRI obtained for investigating mental retardation and developmental abnormalities.
- In addition, due to the development of cytogenetic analysis, it is possible to determine the position and size of the deleted region in the short arm of chromosome 5.6 Considering the correlation between clinical symptoms and the location of the deletion on the chromosome, it is meaningful to pinpoint the region of deletion in this syndrome.6 The regions for facial anomalies, cat-like cry and speech are located on the terminal part from 5p11 to 5p15.33.6 It was confirmed that there was loss of around 24Mb from the p15.33 to p14.2 region of the short arm of chromosome 5 by performing an additional microarray inspection in this infant. Accordingly, it was found that the location of the deletion on the chromosome in this case affected the critical symptoms such as high-pitched cries, mental retardation, developmental delay, and abnormality of microcephaly. Therefore, considering that the bigger the size of the terminal deletion the higher the severity of the clinical manifestation and psychomotor retardation, cytogenetic molecular analysis may be necessary to diagnose the syndrome and to confirm the deletion site of the chromosome as well as to obtain the prognosis.
- In the early publications, the pathogenesis of the typical cat-like cry was thought to be associated with a small, flappy, curved epiglottis and narrow, hypoplastic, quadrangular larynx.6 However, in one study of 23 patients with 5p deletion, the pathogenesis of a high-pitched cry was considered to be associated with brain-based brainstem malformation.6 There have been some reports suggesting that the specific cranial base region develops around the notochordal area from where the rhombencephalic-derived brainstem, pons, and cerebellum develop dorsally while the neurons to the larynx migrate ventrally.6 This cranial base mechanism explains that the high-pitched cry is associated with hypoplasia of the brainstem like pontine. In other words, it was suggested that the characteristic cat-like cry was caused by the variation in anomalies of the brain structures and larynx, which occurred from the developmental derangements of the notochord around the area of the specific cranial base region.9 Considering that the larynx is involved in the development of the cranial base,6 it can be assumed that the variation in anomalies of the cranial base region is the likely reason why this infant showed feeding hypoxemia and bottle feeding problem.
- This syndrome also presents with other clinical features such as microcephaly and mental retardation.89 CdCS presents, although not very often, with urinary abnormalities including ectopic hypoplasia or horseshoe kidney.5 Also, a slightly small sized kidney for his age was observed in this infant patient. Among the cardiac congenital defects related to CdCS, ductus associated with a septal ventricular defect has often been observed, while ductus with a septal atrial defect has been observed less frequently.47 Although CdCS uncommonly presents with deafness,5 hearing test results were normal in this infant's case. Nonetheless, further follow-up hearing test will be necessary due to the fear of hearing loss afterwards.
- The postnatal treatment of preterm infants is focused on the main symptoms of respiratory distress and acute diseases through intubation and ventilator therapy. However, considering that a chromosomal disease could also be found in premature infants afterwards, when abnormalities and pathognomonic symptoms are recognized, diagnostic tools including molecular cytogenetic analysis and cranial MRI are needed. Therefore, chromosomal examination and assessment of further diagnoses should be conducted so as not to miss the CdCS in case pontine hypoplasia on cranial MRI is observed in premature babies or young children with mental retardation and delayed development. Taking all the above considerations into account, it is important that neonatal physicians should keep in mind that the symptoms of chromosomal diseases can be easily overlooked during the early period of life in preterm infants and neonates, focusing on the acute disease treatment. They should also remember that immediate chromosome analysis and active neuroradiological procedures need to be performed on suspicion of CdCS.
DISCUSSION
- 1. Garofalo E, Minerva A. Crying cat syndrome in a premature infant. Clin Pediatr (Bologna) 1970;52:80–91.PubMed
- 2. Beaney A. Case study: a premature baby with cri-du-chat syndrome. Midwives Chron 1976;89:158–160.PubMed
- 3. Kim CH, Cho EH, Shin SM, Kim JS. A Case of Cat Cry Syndrome. J Korean Pediatr Soc 1975;18:532–536.
- 4. Lejeune J, Lafourcade J, Berger R, Vialatte J, Boeswillwald M, Seringe P, et al. 3 cases of partial deletion of the short arm of a 5 chromosome. C R Hebd Seances Acad Sci 1963;257:3098–3102.PubMed
- 5. Rodríguez-Caballero A, Torres-Lagares D, Rodríguez-Pérez A, Serrera-Figallo MA, Hernández-Guisado JM, Machuca-Portillo G. Cri du chat syndrome: a critical review. Med Oral Patol Oral Cir Bucal 2010;15:e473–e478.PubMed
- 6. Kjaer I, Niebuhr E. Studies of the cranial base in 23 patients with cri-du-chat syndrome suggest a cranial developmental field involved in the condition. Am J Med Genet 1999;82:6–14.ArticlePubMed
- 7. Niebuhr E. The Cri du Chat syndrome: epidemiology, cytogenetics, and clinical features. Hum Genet 1978;44:227–275.ArticlePubMedPDF
- 8. Jones KL. Smith's recognizable Patterns of Human Malformation. 6th ed. Elsevier Saunders: Philadelphia; 2006.
- 9. Hong JH, Lee HY, Lim MK, Kim MY, Kang YH, Lee KH, et al. Brain Stem Hypoplasia Associated with Cri-du-Chat Syndrome. Korean J Radiol 2013;14:960–962.ArticlePubMedPMC
- 10. Barkovich AJ, Patay Z. Metabolic and neurodegenerative disorders primarily involving the cerebellum. In: Barkovich AJ, Raybaud C, editors. Pediatric Neuroimaging. Philadelphia: Lippincott Williams & Wilkins; 2012. p. 210–212.
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Brain MRI Findings of the Cri-Du-Chat Syndrome: A Case Report and Summary
Jin Sol Choi, Eun Ae Yoo, Jin Ok Choi, Soo Jung Kim
Journal of the Korean Society of Radiology.2020; 81(4): 979. CrossRef

 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader Cite
Cite