Articles
- Page Path
- HOME > Kosin Med J > Volume 35(2); 2020 > Article
-
Original Article
Effects of White-coat Hypertension on Heart Rate Recovery and Blood Pressure Response during Exercise Test - Sol Jin, Jung Ho Heo, Bong Jun Kim
-
Kosin Medical Journal 2020;35(2):89-100.
DOI: https://doi.org/10.7180/kmj.2020.35.2.89
Published online: December 31, 2020
1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea
2Department of Cardiology, Kosin University College of Medicine, Busan, Korea
- Corresponding Author: Jung Ho Heo, Department of Cardiology, Kosin University College of Medicine, 262, Gamcheon-ro, Seo-gu, Busan 49267, Korea, Tel: +82-51-990-6460, Fax: +82-51-990-3049, E-mail: duggymdc@gmail.com
• Received: April 3, 2020 • Revised: May 28, 2020 • Accepted: June 26, 2020
Copyright © 2020 by Korean Association of Medical Journal Editors
Articles published in Kosin Medical Journal are open-access, distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,783 Views
- 8 Download
- 1 Crossref
Abstract
-
Objectives
- White-coat hypertension is defined as high blood pressure (BP) on clinical assessment but normal BP elsewhere or on ambulatory measurement. Autonomic dysfunction may be one of the mechanisms causing white-coat hypertension. Slowed heart rate recovery and excessive BP response during exercise test are associated with autonomic dysfunction. The purpose of this study was to determine the association between white-coat hypertension and abnormal autonomic nervous system response.
-
Methods
- We assessed 295 patients stratified into three groups via 24hr ambulatory BP monitoring, following 2017 ACC/AHA guidelines: normal BP group, white-coat hypertension group, and a hypertension group. We analyzed medical history, blood test, echocardiography, 24hr ambulatory BP monitoring, and exercise test data.
-
Results
- There was no difference in basement characteristics and echocardiography among the groups. Blunted heart rate recovery of each group showed a significant difference. Control group had 0% blunted heart rate recovery, but 33.3% in white coat group and 27.6% in true hypertension group (P < 0.001). Also, in the control group, 4.5% showed excessive BP response, but 31.5% in the white coat hypertension group and 29.3% in the true hypertension group (P < 0.001). Excessive BP response during the exercise test or blunted heart rate recovery, which is an indicator of autonomic nervous system abnormality, was more common in the hypertensive group and white-coat hypertension group than in the normal BP group.
-
Conclusions
- These results confirmed that white-coat hypertension has an autonomic nervous system risk. Therefore, white-coat hypertension can be a future cardiovascular risk factor.
- This is a cross-sectional, single center, case control study. We retrospectively reviewed 295 patients who underwent Treadmill test and 24-hour ambulatory BP monitoring (ABPM) between January 2008 and February 2015. Inclusion criteria were: 18–80 years of age, normal renal function, and for women to be on a regular menstrual cycle. Exclusion criteria were: any systemic disease such as significant liver disease, neurologic disorders or malignant disease, or secondary hypertension.
- Patients were classified according to the ABPM, following diagnostic criteria suggested by the 2017 ACC/AHA guidelines.13 Hypertension group was meeting one or more of these criteria; a 24-hour mean of 125/75 mmHg or above, daytime (awake) mean of 130/80 mmHg or above or Nighttime (asleep) mean of 110/65 mmHg or above. White coat hypertension is defined as having elevated clinic blood pressure, at or above threshold for hypertension without elevated out-of-office blood pressure, below threshold for hypertension. Patients not included in the two groups were classified as normotensive control. After all, patients were classified as 174 patients with true hypertension, 54 patients with white-coat hypertension, and 67 normotensive controls were included.
- Demographic characteristics recorded at the first visit included age, sex, height, weight, current medications, smoking history, and other comorbidities. Blood was drawn for the measurement of total serum cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) cholesterol, triglycerides, blood glucose, creatinine, uric acid, and high sensitivity C-reactive protein (Hs-CRP). Body mass index (BMI) was calculated as the ratio of weight in kilograms to height in square meters. This study was approved by the Kosin University International Review Board.
- - BP measurement and 24-h ambulatory BP monitoring
- Office BP measurements were performed twice at 5-min intervals using a mercury sphygmomanometer. Noninvasive 24-h ABPM was performed on each patient’s non-dominant arm using an automatic oscillometric device (TONOPORT V, PAR Medizintechnik, Berlin, Germany) on a normal working day. Patients were asked to refrain from performing fast exercises. All subjects were instructed to rest or sleep between 10:00 PM and 7:00 AM (nighttime) and continue their usual activities between 7:00 AM and 10:00 PM (daytime). The accuracy of the device was checked against the standard auscultatory method of measuring BP to ensure that the difference in BP measurements between methods did not exceed 5 mmHg. The device was set to obtain BP readings at 20-min intervals during the daytime and at 40-min intervals during the nighttime. Only 24-h recordings that included at least 80% successful recordings were accepted as valid. Each ABPM dataset was first automatically scanned to remove artifactual readings according to preselected editing criteria. The following ABPM parameters were evaluated: 24-h mean SBP and diastolic BP (DBP) levels, daytime mean SBP and DBP levels, nighttime mean SBP and DBP levels, and BP variability assessed with standard deviation (SD).
- - Treadmill test
- All patients underwent symptom-limited exercise stress testing (GE CASE T2100; GE Medical Systems, Milwaukee, WI, USA) according to the protocol by Bruce et al..14 BP was measured using an automated BP monitor (Suntech Tango; Suntech Medical, Morrisville, NC, USA) throughout the treadmill test using the same arm as was used to measure the resting BP. Twelve-lead electrocardiography was monitored continuously and printed at a paper speed of 25 mm/s; measurements of HR and BP were recorded at the end of each 3-min stage at peak exercise and at 1-min and 2-min intervals throughout recovery.
- Treadmill test was continued until the participants felt intolerable fatigue or their HR exceeded 95% of estimated maximal HR (220 bpm, age). The total exercise time was also recorded. EBPR was defined as the peak exercise SBP of ≥ 210 mmHg in men and ≥ 190 mmHg in women.15 Functional capacity was estimated in metabolic equivalents (METs) on the basis of speed and grade of the treadmill.16 During the recovery phase, the subjects continued to walk for 60s at a speed of 1.5 mph, and then they sat down for 3min with continued monitoring of BP, HR, and heart rhythm. HRR was defined as peak heart rate minus heart rate after a 1-min recovery; abnormal HRR was defined as ≤ 12 beats/min.17
- - Echocardiographic measurement
- Standard 2-dimensional echocardiography was performed on all subjects lying in the left lateral decubitus position using a 3.5-MHz transducer (Philips iE33, Philips Medical Systems, Bothell, WA, USA) and the echocardiography examiners were blinded to patient information. Measurements of thickness of the interventricular septum and posterior wall, diameter of the left ventricle (LV) cavity, and the LV mass index (LVMI) were performed according to criteria outlined by the American Society of Echocardiography.18 Pulsed wave Doppler of transmitral LV inflow was performed in the apical four-chamber view with the sample volume placed at the level of the mitral valve tips; doppler variables were analyzed during three consecutive beats. The following measurements of global LV diastolic function were determined: peak early (E) and late (A) diastolic mitral flow velocity, E/A ratio, and early (Ea) diastolic mitral annular velocity.
- - Statistical analysis
- Statistical analyses were performed using the commercially available computer program SPSS 18.0 for Windows (IBM, Chicago, IL, USA). Data are presented as mean ± standard deviation for continuous variables and percentage (%) if the data are categorical. The Mann–Whitney U test was used for continuous variables and the Chi-square test was used for categorical data. The normality of data was tested using the Kolmogorov–Smirnov test. For all tests, the significance level was set to P < 0.05.
MATERIALS AND METHODS
- 1. Characteristics & Medical history
- From January 2008 to February 2015, 295 patients were enrolled in this study. Patients were divided into control, white-coat hypertension, and true hypertension groups.
- At baseline, compared to the normotensive control group, the white-coat hypertension group showed higher BMI, SBP, and DBP. BMI was lowest in the control group at 23.2 kg/m2 and highest at 24.9 kg/m2 in the true hypertension group (P = 0.001). SBP/DBP was lowest in the control group and highest in the white-coat group (P < 0.001). There was no difference in age, sex, heart rate, current smoking, diabetes, and dyslipidemia among the groups (Table 1).
- There was no statistically significant difference in medical history with regard to aspirin, RAS blocker, calcium channel blocker, diuretics, beta blocker, and statin intake.
- 2. Laboratory test
- In laboratory data, uric acid, eGFR, Hs-CRP, WBC, and Platelet levels were similar between the groups. Mean total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides were the highest in the true hypertension group, but the difference was not significant.
- 3. 24-h ambulatory HR & BP monitoring
- 24-h ambulatory BP monitoring was done. As a result, 24-h heart rate and daytime and night time heart rate were higher in the true hypertensive group than in the control and white hypertensive groups (P = 0.001). Additionally, the 24-hour systolic blood pressure/diastolic blood pressure was 142/91 in the true hypertension group, but 119/75 and 122/75 in the control and white-coat hypertension groups respectively. This represents a significant difference in BP (P < 0.001). Day time SBP, DBP, SBP-SD, DBP-SD, MBP, and MBP-SD showed meaningful differences between control, white-coat hypertension, and true hypertension groups. All results were highest in the true hypertension group, and the same appeared in the night time (P < 0.001) (Table 2).
- 4. Echocardiography
- Echocardiography was performed on all patients and compared according to hypertension type. Ejection fraction (EF), diastolic left ventricular internal dimension (LVIDd), systolic left ventricular internal dimension (LVIDs), left atrium (LA) diameter, LA volume, E velocity, A velocity, and E/Ea shows no significant difference. The white-coat hypertension and true hypertension groups have significantly higher epicardial fat thickness value, and the left ventricle posterior wall diameter on echocardiography was thicker in the true hypertension group than in the control and white hypertensive groups (P < 0.001).
- 5. Exercise test
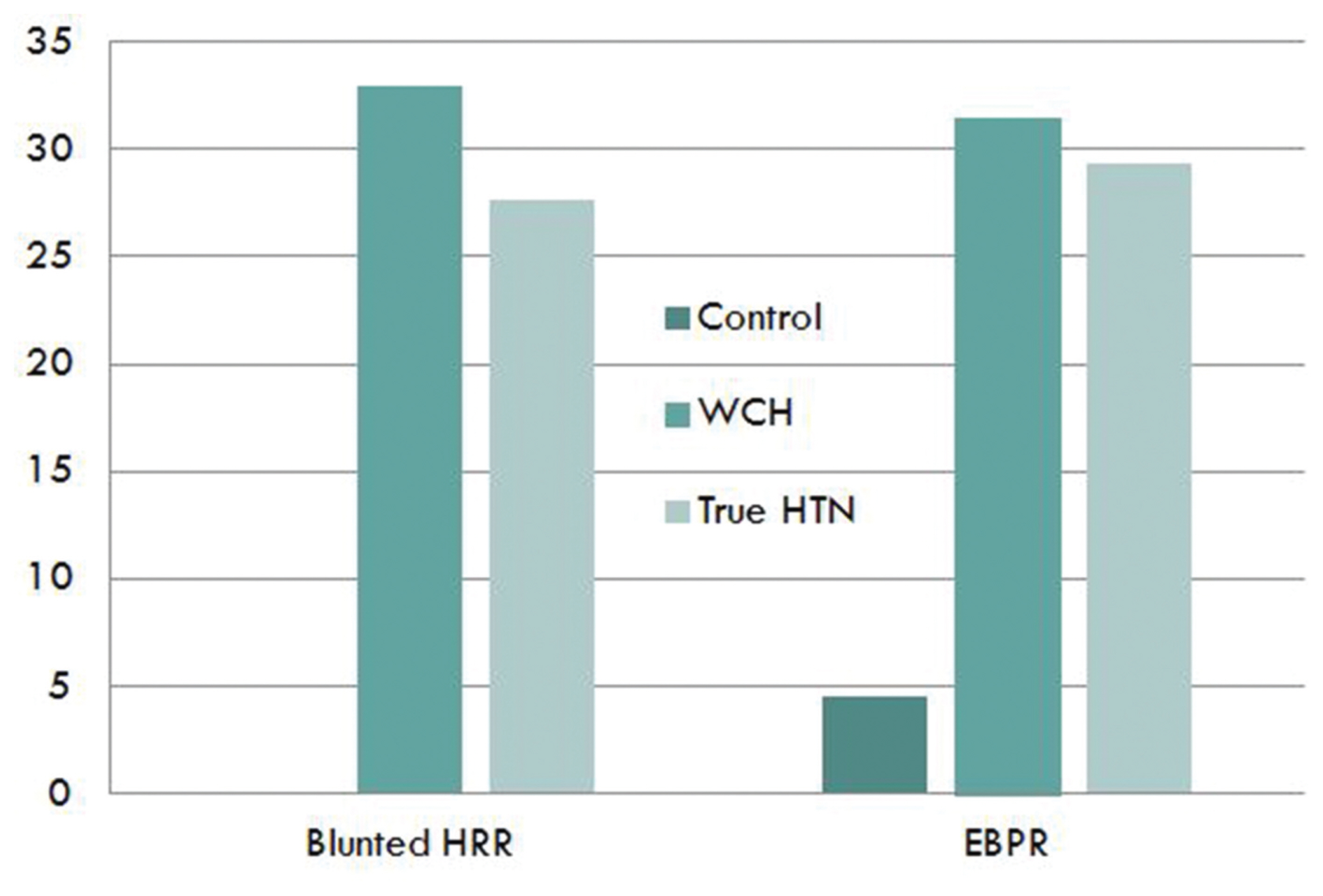
- Compared to the normotensive group, there is no difference in exercise time, rest heart rate, max heart rate, QT, and QTC. However, HRR was significantly lower in the white-coat hypertension and true hypertension groups when compared to the normotensive group (P < 0.001). HRR between groups showed a significant difference. The control group had 0 blunted HRR, but 33.3% (18 patients) in the white-coat group and 27.6% (48 patients) in the true hypertension group. This shows that the prevalence of blunted HRR was significantly higher in the white-coat hypertension and true hypertension groups than the normotensive group (Table 3).
- In the control group, 4.5% (3 patients) showed EBPR, but 31.5% (17 patients) in the white-coat hypertension group and 29.3% (51 patients) in the true hypertension group showed EBPR. The white-coat hypertension group showed the highest percentage of EBPR among all the groups.
- In conclusion, the white-coat hypertension group and the true hypertension group showed significantly low HRR and higher prevalence of blunted HRR (Fig. 1).
RESULTS
- The main finding of our study is that the parameter related with the nervous system responses to exercise such as HRR and EBPR are significantly different in the white-coat hypertension group from the normotensive group. These abnormal responses are similar to that of the true hypertension group. These results could represent another pathomechanism of white-coat hypertension.
- The prognostic impact of white-coat hypertension is still a matter of debate and controversy.19 Accumulating evidence focusing on the association of white-coat hypertension with subclinical target organ damage and, more importantly, incident cardiovascular disease suggests that the risk is intermediate between normotension and sustained hypertension. Pierdomenico et al.20 stated that the white-coat hypertension group did not differ significantly from the normotensive group in terms of risk of a cardiovascular event, but because the white-coat hypertension group patients have much greater rate of antihypertensive treatment at follow up than the normotensive group, the results are not conclusive. The risk of cardiovascular event in white-coat hypertension was shown in a study by Verdecchia et al.21 to be dependent on baseline value of BP on ABPM. In this study, data identified that the incidence of cardiovascular events tends to increase consistently above the daytime BP cut-off value of 130/80 mmHg. Similarly, as per the International Database of Home BP in relation to Cardiovascular Outcomes (IDHOCO), among the untreated subjects, cardiovascular risk was significantly higher in the white-coat hypertension group than in the normotensive group.22 Furthermore, a meta-analysis of 14 studies with 29,100 participants performed by Briasoulis et al..23 showed that the incidence of overall cardiovascular events was 6.0% in the white-coat hypertension subjects when compared to 4.0% in the normotensive subjects, thus meaning a 73% increased risk (P < 0.001). The risk for fatal cardiovascular events was increased even more, the incidence being 4.0% and 1.2% in the white-coat hypertension and normotensive groups, respectively.
- There are many studies on the identification of the mechanism of white-coat hypertension, but these are not clear. The current study investigates whether individuals with white-coat hypertension have abnormal autonomic-cardiac regulation or impaired vascular function, similar to that observed in sustained or persistent hypertension. Sustained hypertension is associated with sympathetic predominance or diminished parasympathetic nervous system influences on heart rate, or both.3, 24 Such “autonomic dysregulation” may be related to hypertension by increasing cardiac output, vascular resistance, and salt retention. Parasympathetic and sympathetic cardiac nervous system functions can be quantified noninvasively through spectral analysis of variability in heart rate.25 Generally, an increase in HR during exercise occurs as a result of combined sympathetic activation and parasympathetic withdrawal.26 In contrast, parasympathetic reactivation is the principal determinant of decreased HR during early recovery. Given the prognostic significance of diminished parasympathetic tone at rest, post-exercise HRR measurement is a noninvasive procedure that can be used to assess parasympathetic activation.27 HRR is simple to calculate using the data obtained from standard exercise tests; moreover, because it does not require 24-h Holter monitoring or baro-reflex sensitivity testing, HRR may be valuable for assessing the risk in routine clinical practice, including exercise test.
- EBPR is also related with the risk of future hypertension and cardiovascular mortality.28 We observed that the white-coat hypertension group had a higher prevalence of EBPR, suggesting that white-coat hypertension might present due to the pathomechanism of impaired vascular response. A recent study by Androulakis el al. showed that the white-coat hypertension group presented with worse endothelial function, more pronounced arterial stiffness, and LVH.29 BP reflects the changes in cardiac output during exercise or recovery that can lead to changes in SBP. Cardiovascular reactivity to both isometric and dynamic exercise has been shown to be one of the most important markers for predicting hypertension. Impaired vascular function, including increased arterial stiffness and abnormal endothelial function, is associated with increased exercise BP response.30 Among the parameters of BP response, exercise BP response is an important marker of cardiovascular risk that is associated with cardiovascular mortality. In particular, EBPR is a significant predictor of cardiovascular events and for new onset of resting hypertension.
- This study has several limitations. First, this is a retrospective study. Second, the cross-sectional study design eliminated our ability to determine causal relationships. Third, this study was performed at a single center, and relatively small number of subjects. So it is possible that biases existed with respect to patient referral and population sampling. In order to overcome this limitation, it is thought that additional large randomised control studies will be needed. At last, our patients were treated by various medications that may have had some effect on BP. At base line, the normotensive group patients also were on RAS blockers, beta blockers, or Calcium channel blockers due to cardiovascular disease or renal disease. These medications might have had an impact on the BP variability and HRR.
- Our findings suggest that autonomic dysregulation assessed by HRR and vascular dysfunction assessed by EBPR have a proportional relationship with white-coat hypertension like true hypertension. The presence of blunted HRR and EBPR showed significant association in the white-coat hypertension group when compared to the normotension group. This means that autonomic dysregulation and vascular dysfunction could be the pathomechanism of white-coat hypertension, and white-coat hypertension is a risk for a cardiovascular event. Based on these study findings, more attention is to be given to the role of white-coat hypertension in cardiovascular target organ damage, and more study in this area is warranted.
DISCUSSION
Fig. 1The percentage of blunted heart rate recovery and exaggerated blood pressure response to exercise according to blood pressure pattern.


Table 1Baseline characteristics
Table 2Clinicopathogenic features of papillary thyroid carcinomas patients analyzed in this study
Table 3Parameters of the exercise test
- 1. Kim KI, Ihm SH, Kim GH, Kim HC, Kim JH, Lee HY, et al. 2018 Korean society of hypertension guidelines for the management of hypertension: part III-hypertension in special situations. Clin Hypertens 2019;25:19.ArticlePubMedPMC
- 2. Mariampillai JE, Engeseth K, Kjeldsen SE, Grundvold I, Liestøl K, Erikssen G, et al. Exercise systolic blood pressure at moderate workload predicts cardiovascular disease and mortality through 35 years of follow-up in healthy, middle-aged men. Blood pressure 2017;26:229–36.ArticlePubMed
- 3. Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res 2014;114:1804–14.ArticlePubMed
- 4. Miyai N, Arita M, Morioka I, Takeda S, Miyashita K. Ambulatory blood pressure, sympathetic activity, and left ventricular structure and function in middle-aged normotensive men with exaggerated blood pressure response to exercise. Med Sci Monit 2005;11:Cr478–84.PubMed
- 5. Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation 1999;99:1831–6.ArticlePubMed
- 6. Lima SG, Albuquerque MF, Oliveira JR, Ayres CF, Cunha JE, Oliveira DF, et al. Exaggerated blood pressure response during the exercise treadmill test as a risk factor for hypertension. Braz J Med Biol Res 2013;46:368–47.ArticlePubMedPMC
- 7. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341:1351–7.ArticlePubMed
- 8. Huang PH, Leu HB, Chen JW, Cheng CM, Huang CY, Tuan TC, et al. Usefulness of attenuated heart rate recovery immediately after exercise to predict endothelial dysfunction in patients with suspected coronary artery disease. Am J Cardiol 2004;93:10–3.ArticlePubMed
- 9. Kizilbash MA, Carnethon MR, Chan C, Jacobs DR, Sidney S, Liu K. The temporal relationship between heart rate recovery immediately after exercise and the metabolic syndrome: the CARDIA study. Eur Heart J 2006;27:1592–6.ArticlePubMed
- 10. Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. Jama 2000;284:1392–8.ArticlePubMed
- 11. Franklin SS, Thijs L, Hansen TW, O’Brien E, Staessen JA. White-coat hypertension: new insights from recent studies. Hypertension 2013;62:982–7.ArticlePubMed
- 12. Neumann SA, Jennings JR, Muldoon MF, Manuck SB. White-coat hypertension and autonomic nervous system dysregulation. Am J Hypertens 2005;18:584–8.ArticlePubMed
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248.PubMed
- 14. Bruce RA, Gey GO Jr, Cooler MN, Fisher LD, Peterson DR. Seattle heart watch: Initial clinical, circulatory and electrocardiographic responses to maximal exercise. Am J Cardiol 1974;33:459–69.ArticlePubMed
- 15. Campbell L, Marwick TH, Pashkow FJ, Snader CE, Lauer MS. Usefulness of an exaggerated systolic blood pressure response to exercise in predicting myocardial perfusion defects in known or suspected coronary artery disease. Am J Cardiol 1999;84:1304–10.ArticlePubMed
- 16. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 Guideline Update for Exercise Testing: Summary Article. Circulation 2002;106:1883–92.ArticlePubMed
- 17. Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003;42:831–8.ArticlePubMed
- 18. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: The framingham heart study. Am J Cardiol 1987;59:956–60.ArticlePubMed
- 19. Faria J, Mesquita-Bastos J, Bertoquini S, Silva J, Barbosa L, Polonia J. Long-term cardiovascular risk of white-coat hypertension with normal night-time blood pressure values. Blood Press Monit 2019;24:59–66.ArticlePubMed
- 20. Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens 2011;24:52–8.ArticlePubMed
- 21. Verdecchia P, Angeli F, Gattobigio R, Borgioni C, Castellani C, Sardone M, et al. The clinical significance of white-coat and masked hypertension. Blood Press Monit 2007;12:387–9.ArticlePubMed
- 22. Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension 2014;63:675–82.ArticlePubMed
- 23. Briasoulis A, Androulakis E, Palla M, Papageorgiou N, Tousoulis D. White-coat hypertension and cardiovascular events: a meta-analysis. J Hypertens 2016;34:593–9.PubMed
- 24. Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 1998;16:1979–87.ArticlePubMed
- 25. Mark AL. The sympathetic nervous system in hypertension: a potential long-term regulator of arterial pressure. J Hypertens 1996;14:S159–65.
- 26. Javorka M, Zila I, Balhárek T, Javorka K. Heart rate recovery after exercise: relations to heart rate variability and complexity. Braz J Med Biol Res 2002;35:991–1000.ArticlePubMed
- 27. Buchheit M, Papelier Y, Laursen PB, Ahmaidi S. Noninvasive assessment of cardiac parasympathetic function: postexercise heart rate recovery or heart rate variability? Am J physiol Heart Circ Physiol 2007;293:H8–H10.ArticlePubMed
- 28. Jae SY, Fernhall B, Heffernan KS, Kang M, Lee MK, Choi YH, et al. Exaggerated blood pressure response to exercise is associated with carotid atherosclerosis in apparently healthy men. J Hypertens 2006;24:881–7.ArticlePubMed
- 29. Androulakis E, Papageorgiou N, Lioudaki E, Chatzistamatiou E, Zacharia E, Kallikazaros I, et al. Subclinical Organ Damage in White-Coat Hypertension: The Possible Role of Cystatin C. J Clin Hypertens (Greenwich) 2017;19:190–7.ArticlePubMed
- 30. Thanassoulis G, Lyass A, Benjamin EJ, Larson MG, Vita JA, Levy D, et al. Relations of exercise blood pressure response to cardiovascular risk factors and vascular function in the Framingham heart study. Circulation 2012;125:2836–43.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Study on Maximal Oxygen Uptake of Respiration and Heart Rate in Exercise Training Based on Regression Equation
Yongqing Liang, Qiufen Yu, Balakrishnan Nagaraj
Journal of Healthcare Engineering.2022; 2022: 1. CrossRef

 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite