Articles
- Page Path
- HOME > Kosin Med J > Volume 37(3); 2022 > Article
-
Original article
A novel technique for transurethral vesicovaginal fistula tract resection followed by transvaginal fistula repair: a two-step procedure -
Soodong Kim1
 , Heejong Jeong2
, Heejong Jeong2 , Wonyeol Cho1
, Wonyeol Cho1
-
Kosin Medical Journal 2022;37(3):236-241.
DOI: https://doi.org/10.7180/kmj.22.124
Published online: September 27, 2022
1Department of Urology, Dong-A University Hospital, Busan, Korea
2Department of Urology, Wonkwang University Hospital, Iksan, Korea
- Corresponding Author: Wonyeol Cho, MD, PhD Department of Urology, Dong-A University Hospital, 26 Daesingongwon-ro, Seo-gu, Busan 49201, Korea Tel: +82-51-240-5446 Fax: +82-51-253-0591 E-mail: urogate@dau.ac.kr
Copyright © 2022 Kosin University College of Medicine.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,042 Views
- 29 Download
Abstract
-
Background
- The principle of treatment for a vesicovaginal fistula (VVF) tract is complete removal of the fistula tract and surrounding scar tissue, followed by anastomosis without tension from surrounding healthy tissue. We present our novel two-step procedure for VVF repair.
-
Methods
- We retrospectively analyzed 12 women, aged 14 to 67 years, who were treated between 2011 and December 2018. Conservative treatments failed, as these patients had complex VVFs. This technique consisted of two steps: first, transurethral resection of the fistula tract and surrounding scar tissue; second, transvaginal repair of the bladder mucosa, bladder muscle, and vaginal mucosa with tensionless anastomosis. If an interposition flap was needed, we used a Martius flap.
-
Results
- The mean operation time was 186.3 minutes (range, 145–320 minutes), and the mean urethral catheter indwelling time was 10 days. Ten patients successfully underwent surgery through a transvaginal approach with no intraoperative or postoperative complications. However, one patient developed peritoneal perforation during transurethral resection of the fistula due to severe granulation tissue formation around the fistula, which prompted conversion to an abdominal approach. In two cases, we used a Martius flap because of the poor tissue condition due to previous radiation therapy and an inflammatory reaction. At a mean follow-up of 37 months (range, 16–51 months), no recurrence of VVF was observed in any patients.
-
Conclusions
- This novel technique for transurethral VVF tract resection followed by transvaginal fistula repair was very safe and effective technique, and this straightforward technique is expected to reduce surgeons’ burden.
- Vesicovaginal fistula (VVF) is an abnormal passage between the vaginal and bladder that results in uncontrolled urinary leakage from the vagina. Although VVF is not a life-threatening condition, VVF has a significantly negative impact on the quality of life. Etiologic factors and prevalence rates of this condition vary from one country to another.
- In developed countries, 90% of VVFs raised from previous pelvic surgery and of these, 70% were due to abdominal hysterectomy for benign disease [1]. The remaining 10% of VVFs occurred because of radiation, infection, foreign bodies, and pelvic malignancies.
- In underdeveloped countries, the main cause of VVFs is poor obstetrics and gynecological conditions, so obstetrical injuries are the most common [2].
- Gynecologic operation-related VVFs usually appear approximately 10 days after operation, while radiation-induced fistulas frequently occur many years after treatment [3,4].
- The principle of VVF repair is complete excision of fistula and scar tissue, followed by tensionless anastomosis of well-vascularized clean tissue. This principle is very important for reducing the recurrence rate. Traditionally, for VVF repair, transvaginal approach or transabdominal approach techniques have been used. However, in general, the VVF is located deep in the vagina, making the transvaginal approach difficult to keep this principle. Furthermore, this principle is not simple to follow with the transabdominal approach due to access to the fistula site (e.g., fibrotic tissue condition by radiation, postoperative adhesion) and a higher rate of postoperative complications. In this study, we present our novel two-step procedure of transurethral VVF tract resection followed by the transvaginal fistula repair technique.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board of Dong-A University Hospital (No. 15-008). The informed consent was waived because this design is a retrospective study.
- 1. Patients and study design
- We analyzed 12 women, aged 14 to 67 years, treated between 2011 and December 2018, retrospectively. The data were collected using hospital medical documentation. The study was conducted after the approval of the institutional review board.
- For assessing of characteristics of the fistula (localization, number, and size), we evaluated surgical history, vaginal examination, cystoscopy, and computed tomography, preoperatively [5]. Fistulas found at sites other than the bladder (ureterovaginal fistula or urethrovaginal) were excluded.
- Classically, VVFs were classified as simple and complex [6]. Simple VVFs were defined as small (≤0.5 cm) and single non-radiated fistulas. Complex VVFs were classified as medium (0.5–2.5 cm), large (≥2.5 cm), multiple, and recurrent fistulas. All patients were conservatively managed with continuous drainage through a Foley catheter for 2 months in anticipation of spontaneous healing. These conservative treatments failed as every patient had complex VVF. Clinical success was defined as postoperative removal of the Foley catheter, no further urine leakage, and no recurrence during the follow-up period.
- 2. Surgical technique
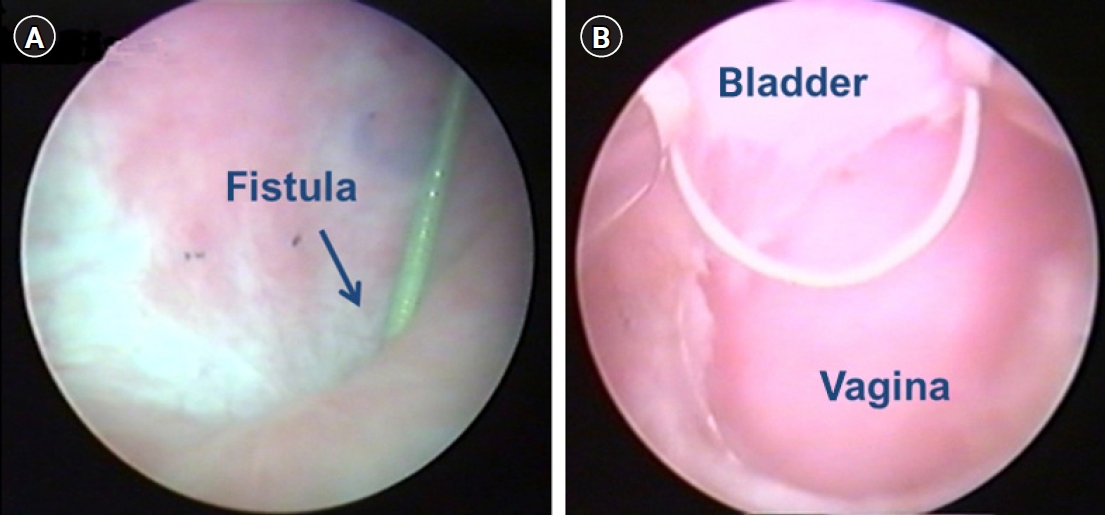
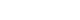
- The surgical technique consists of two steps. The first step involves complete transurethral resection of the fistula tract and surrounding scar tissue. All patients underwent general anesthesia or spinal anesthesia. The patients were operated in the dorsal lithotomy position. Firstly, fistula resection is performed using a 24-Fr resectoscope under continuous flow. We used a 30° lens and wire cutting loop. To achieve a clear resection field, a continuous flow sheath is very important (Fig. 1). During resection with a cutting loop should be performed in a systematic, piecemeal manner, aiming at the complete resection of the granulation tissue around the fistula. Practically, resection should begin bladder mucosa toward the deeper layers of vagina. If bleeding occurs, electrocautery should be used minimally to save vascularity. After endoscopic surgery, we placed a Foley catheter and changed to conventional transvaginal surgical approach. The second step involves transvaginal tensionless repair of bladder mucosa followed by bladder muscle and vaginal mucosa repaired layered closure. If an interposition flap is needed due to poor tissue condition, the Martius flap could be used.
- After fat pad was harvested from the labia major, pulled up to the suture line through deep tunneled vaginal mucosa.
Methods
- A total of 12 patients underwent this novel transurethral VVF tract resection followed by transvaginal fistula repair technique. The mean age of patients was 47.1 years (range, 14–67 years) and the mean follow-up period was 37 months (range, 16–51 months). Based on the surgical history, four patients had a radical hysterectomy due to cervical cancer, six had a laparoscopic hysterectomy due to uterine myoma, and one underwent a hypogastric artery ligation due to vaginal bleeding following a cervical biopsy. The last patient had no surgical history, but she inserted a foreign body into the vagina (Table 1). Three patients were second trial of VVF repair.
- The VVF was located high supra-trigonal in all patients, and its size ranged from 0.7 to 1.6 cm (mean, 1.1 cm), indicating complex VVFs.
- The mean operation time was 186.3 minutes (range, 145–320 minutes), and the mean urethral catheter indwelling time was 10 days. In 10 patients, the operation was conducted successfully through a transvaginal approach with no intraoperative or postoperative complications.
- However, one patient developed a peritoneal perforation during transurethral resection of the fistula due to severe granulation tissue formation around the fistula and was converted to an abdominal approach.
- In two cases, we used an interposition flap by Martius flap for VVF repair because of poor tissue condition due to previous radiation therapy and inflammatory reaction. At a mean follow-up of 37 months (range, 16–51 months), there was no recurrence observed in all 12 patients.
Results
- The treatment of VVF is remained a challenge to the surgeon because there were several controversies still exist. The most cited controversies are about the operation timing, ideal surgical approach, and need for adjuvant measures. A trial of conservative therapy was conducted with proper and undisturbed bladder drainage for the small fistulas. The success rate of conservative therapy was limited success (7%–12.5%) in only selected cases [7,8]. After conservative therapy, there is persistent urinary leakage in the vagina, surgical correction treatment is necessary.
- Several operation techniques exist, such as endoscopy, laparoscopy, robot-assisted, and conventional open surgery through the vagina or abdomen [9-11]. However, the ideal approach for VVF repair remains controversial. The transabdominal approach is adopted for the repair of supra-trigonal vesicovaginal, ureterovaginal, or vesicouterine fistulas, and if bladder augmentation is required; the omentum is used as a flap. The transvaginal approach is preferred for repair wherever possible, and a Martius flap is usually used from the subcutaneous fat of the labium [12,13]. The success rate has varied between 75% and 95% with these various techniques [9-12,14-17]. In this study, we achieved clinical success in all patients with no recurrence. In addition, two patients needed an interposition flap (Martius flap) due to a previous radiation treatment history and poor tissue condition due to an inflammatory reaction.
- The first attempt to treat VVF using an endoscopic procedure demonstrated transurethral pointed electrode VVF repair. It was feasible in patients with multiple, small VVFs [18].
- For a successful operation, several principles must be satisfied as follows. First step involves adequately mobilizing the bladder from the vagina, exposing the fistula tract. And complete remove surrounding scar tissues while revealing healthy tissue edges. Second, closing the bladder and vagina in separate layers in water-tight manner without tension with healthy tissues. If proper healthy tissues were not secured, interposition flap could be used between the bladder and vagina suture lines. Third, urine natural drainage should be maintained, postoperatively [15,19,20].
- In this study, before repairing the fistula, we effectively removed the VVF and scar tissues on the bladder and vagina by endoscopic resection. Then, we closed the bladder and vagina with viable clean tissue through transvaginal approach. We consider anastomosis with viable clean tissue a crucial step for a VVF repair.
- The operation times reported in the literature ranged from 70 to 280 minutes [21-23]. Our mean operative time was 186.3 minutes which seems consistent with the most reported cases. Operation time is affected by several factors. Representative factors are fistula location, onset time, previous surgical history, failed fistula repair history and experience and skill of the surgeon. In our case, three patients had a previous history of failed fistula repair. In one of the cases, peritoneal perforation occurred during transurethral resection of the VVF. Therefore, the patient converted to open surgery, and VVF was completely repaired.
- The time of surgical repair of VVF is still controversial. Classically, there should be 3 to 6 months following the onset of the VVF before surgical repair to allow the surgical inflammatory reaction may subside. The overall success rate of VVF repair, including those for whom repairs were done within 3 months post-injury, and those for whom the repairs were intentionally delayed, ranged from 86% to 100% [10,24-27]. In our study, transvaginal VVF was repaired at least 2 months after the onset of symptom or the initial surgery to allow subsiding of the infection or inflammation at the fistula site. According to the literature, urethral catheter is removed at 10 to 28 days [21,23]. In our case, the urethral catheter was kept for 10 days in all patients. Before catheter removal, all patients were checked cystography to confirm a complete closure of the VVF. No leakage was observed in all patients, and they were dry after catheter removal for follow-up periods.
- A limitation of this study includes a single-center, retrospective trial, which may decrease the quality of evidence. Another limitation is the small sample size of patients. Despite this limitation, transurethral resection of VVF and surrounding scar tissue is simple under clear vision. As a result, the success rate of the VVF repair may be improved.
- In conclusion, this novel transurethral VVF tract resection followed by transvaginal fistula repair technique was a very safe and effective technique. And this easy technique is expected to reduce the burden of surgeons.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: WC. Data curation: SK, HJ. Formal analysis: WC, HJ. Investigation: SK. Methodology: WC, HJ. Project administration: SK. Resources: SK. Software: SK. Supervision: WC, HJ. Validation: WC, HJ. Visualization: WC, HJ. Writing - original draft: SK. Writing - review & editing: WC, HJ. Approval of final manuscript: all authors.
Article information
- 1. Stothers L, Chopra A, Raz S. Vesicovaginal fistula. In: Raz S, editors. Female urology. Philadelphia: WB Saunders; 1996. p. 490–506.
- 2. Rackley RR, Appell RA. Vesicovaginal fistula: current approach. AUA Update Ser 1988;17:162–7.
- 3. Carr LK, Webster GD. Abdominal repair of vesicovaginal fistula. Urology 1996;48:10–1.Article
- 4. Margolis T, Mercer LJ. Vesicovaginal fistula. Obstet Gynecol Surv 1994;49:840–7.ArticlePubMed
- 5. Goodwin WE, Scardino PT. Vesicovaginal and ureterovaginal fistulas: a summary of 25 years of experience. J Urol 1980;123:370–4.ArticlePubMed
- 6. Angioli R, Penalver M, Muzii L, Mendez L, Mirhashemi R, Bellati F, et al. Guidelines of how to manage vesicovaginal fistula. Crit Rev Oncol Hematol 2003;48:295–304.ArticlePubMed
- 7. Hilton P, Ward A. Epidemiological and surgical aspects of urogenital fistulae: a review of 25 years' experience in southeast Nigeria. Int Urogynecol J Pelvic Floor Dysfunct 1998;9:189–94.ArticlePubMedPDF
- 8. Hilton P. Vesico-vaginal fistula: new perspectives. Curr Opin Obstet Gynecol 2001;13:513–20.ArticlePubMed
- 9. Hodges AM. The Mitrofanoff urinary diversion for complex vesicovaginal fistulae: experience from Uganda. BJU Int 1999;84:436–9.ArticlePubMed
- 10. Raz S, Bregg KJ, Nitti VW, Sussman E. Transvaginal repair of vesicovaginal fistula using a peritoneal flap. J Urol 1993;150:56–9.ArticlePubMed
- 11. McKay HA. Vesicovaginal fistula repair: transurethral suture cystorrhaphy as a minimally invasive alternative. J Endourol 2004;18:487–90.ArticlePubMed
- 12. Martius H. Die operative Wiederherstellung der vollkommen fehlenden Harnrohre und des Schiessmuskels derselben. Zentralbl Gynak 1928;52:480–6.
- 13. Kumar A, Goyal NK, Das SK, Trivedi S, Dwivedi US, Singh PB. Our experience with genitourinary fistulae. Urol Int 2009;82:404–10.ArticlePubMedPDF
- 14. Arrowsmith S, Hamlin EC, Wall LL. Obstructed labor injury complex: obstetric fistula formation and the multifaceted morbidity of maternal birth trauma in the developing world. Obstet Gynecol Surv 1996;51:568–74.ArticlePubMed
- 15. Ou CS, Huang UC, Tsuang M, Rowbotham R. Laparoscopic repair of vesicovaginal fistula. J Laparoendosc Adv Surg Tech A 2004;14:17–21.ArticlePubMed
- 16. Sims JM. On the treatment of vesico-vaginal fistula. Am J Med Sci 1852;23:59–82.Article
- 17. Iselin CE, Aslan P, Webster GD. Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol 1998;160(3 Pt 1):728–30.Article
- 18. Hong HM, Lee JW, Han DY, Jeong HJ. Vesicovaginal fistula repair using a transurethral pointed electrode. Int Neurourol J 2010;14:65–8.ArticlePubMedPMC
- 19. O'Conor VJ Jr. Review of experience with vesicovaginal fistula repair. J Urol 1980;123:367–9.ArticlePubMed
- 20. Nezhat CH, Nezhat F, Nezhat C, Rottenberg H. Laparoscopic repair of a vesicovaginal fistula: a case report. Obstet Gynecol 1994;83(5 Pt 2):899–901.
- 21. Otsuka RA, Amaro JL, Tanaka MT, Epacagnan E, Mendes JB Jr, Kawano PR, et al. Laparoscopic repair of vesicovaginal fistula. J Endourol 2008;22:525–8.ArticlePubMed
- 22. Shah SJ. Laparoscopic transabdominal transvesical vesicovaginal fistula repair. J Endourol 2009;23:1135–7.ArticlePubMed
- 23. Kapoor R, Ansari MS, Singh P, Gupta P, Khurana N, Mandhani A, et al. Management of vesicovaginal fistula: an experience of 52 cases with a rationalized algorithm for choosing the transvaginal or transabdominal approach. Indian J Urol 2007;23:372–6.ArticlePubMedPMC
- 24. Persky L, Herman G, Guerrier K. Nondelay in vesicovaginal fistula repair. Urology 1979;13:273–5.ArticlePubMed
- 25. Cruikshank SH. Early closure of posthysterectomy vesicovaginal fistulas. South Med J 1988;81:1525–8.ArticlePubMed
- 26. Tancer ML. The post-total hysterectomy (vault) vesicovaginal fistula. J Urol 1980;123:839–40.ArticlePubMed
- 27. Wein AJ, Malloy TR, Carpiniello VL, Greenberg SH, Murphy JJ. Repair of vesicovaginal fistula by a suprapubic transvesical approach. Surg Gynecol Obstet 1980;150:57–60.ArticlePubMed
References
Figure & Data
References
Citations


 KOSIN UNIVERSITY COLLEGE OF MEDICINE
KOSIN UNIVERSITY COLLEGE OF MEDICINE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite